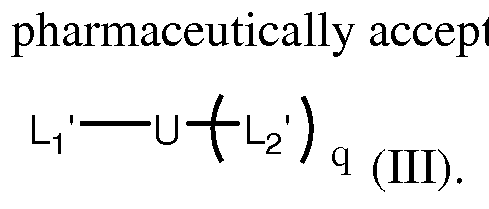WO2014194030A2 - Conjugates comprising cell-binding agents and cytotoxic agents - Google Patents
Conjugates comprising cell-binding agents and cytotoxic agents Download PDFInfo
- Publication number
- WO2014194030A2 WO2014194030A2 PCT/US2014/039919 US2014039919W WO2014194030A2 WO 2014194030 A2 WO2014194030 A2 WO 2014194030A2 US 2014039919 W US2014039919 W US 2014039919W WO 2014194030 A2 WO2014194030 A2 WO 2014194030A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ala
- optionally substituted
- methyl
- independently
- val
- Prior art date
Links
- 0 CC(C(NCC(NCC(NCCCCC(C(NCCC(NCCN(C(CC1**)=O)C1=O)=O)=O)NC(CNC(CNC(C(C)NC(CCCS*)=O)=O)=O)=O)=O)=O)=O)NC(CCCS*)=O Chemical compound CC(C(NCC(NCC(NCCCCC(C(NCCC(NCCN(C(CC1**)=O)C1=O)=O)=O)NC(CNC(CNC(C(C)NC(CCCS*)=O)=O)=O)=O)=O)=O)=O)NC(CCCS*)=O 0.000 description 14
- JWIKQSJBMZGAAW-UHFFFAOYSA-N C=C=[N]=C=[N]=C=[N]=C=N Chemical compound C=C=[N]=C=[N]=C=[N]=C=N JWIKQSJBMZGAAW-UHFFFAOYSA-N 0.000 description 1
- KOHJIKSTBRXNOV-UHFFFAOYSA-N CC(CC(N1I)=O)C1=O Chemical compound CC(CC(N1I)=O)C1=O KOHJIKSTBRXNOV-UHFFFAOYSA-N 0.000 description 1
- MJEKMYYSIWOILD-FIVVQALRSA-N C[C@@H]([C@@H]1O[C@@]1(C)CCC(N(C)c1cc(C/C(/C)=C/C=C/[C@H]([C@@](C2)(N3)O)OC)cc(OC)c1Cl)=O)[C@H]2OC3=O Chemical compound C[C@@H]([C@@H]1O[C@@]1(C)CCC(N(C)c1cc(C/C(/C)=C/C=C/[C@H]([C@@](C2)(N3)O)OC)cc(OC)c1Cl)=O)[C@H]2OC3=O MJEKMYYSIWOILD-FIVVQALRSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6883—Polymer-drug antibody conjugates, e.g. mitomycin-dextran-Ab; DNA-polylysine-antibody complex or conjugate used for therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6889—Conjugates wherein the antibody being the modifying agent and wherein the linker, binder or spacer confers particular properties to the conjugates, e.g. peptidic enzyme-labile linkers or acid-labile linkers, providing for an acid-labile immuno conjugate wherein the drug may be released from its antibody conjugated part in an acidic, e.g. tumoural or environment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Definitions
- ADC Antibody-drug conjugates
- cell binding agent-drug conjugates are emerging as a powerful class of anti-tumor agents with efficacy across a range of cancers.
- the cell binding agent-drug conjugates are commonly composed of three distinct elements: a cell-binding agent (e.g., antibody); a linking component; and a cytotoxic moiety.
- the linking component of ADC is an important element in developing targeted anticancer agents that possess an optimal therapeutic window, i.e., therapeutic activity at a low, non-toxic dose.
- a first embodiment of the invention features a conjugate represented by the following formula, or a pharmaceutically acceptable salt thereof: (I).
- CBA is a cell binding agent
- hi is a spacer
- U is a branched scaffold
- L 2 for each occurrence, is independently a spacer
- D for each occurrence, is independently a cytotoxic drug moiety
- q is an integer from 2 to 5
- w is an integer between 1 and 10.
- a second embodiment of the invention features a cytotoxic compound represented by the following formula, or a salt (e.g., a pharmaceutically acceptable salt) thereof:
- L is a spacer attached to a reactive functional group that can form a covalent bond with a cell-binding agent;
- U is a branched scaffold;
- L 2 for each occurrence, is independently a spacer;
- D for each occurrence, is independently a cytotoxic drug moiety; and
- q is an integer from 2 to 5.
- a third embodiment of the invention features a linker compound represented by the following formula, or a salt (e.g., a table salt) thereof:
- a fourth embodiment of the invention features a modified cell binding agent represented by the following formula, or a salt (e.g. , a pharmaceutically acceptable salt) thereof:
- CBA is a cell binding agent
- h is a spacer
- U is a branched scaffold
- L 2 ' for each occurrence, is independently a spacer attached to a reactive functional group that can form a covalent bond with a cytotoxic agent
- q is an integer from 2 to 5
- w is an integer between 1 and 10.
- composition e.g. , a pharmaceutical composition
- a pharmaceutical composition comprising a conjugate represented by Formula (I), a cytotoxic compound represented by Formula (II), or a salt (e.g. , a pharmaceutical acceptable salt) thereof.
- the composition may also include a carrier (e.g. , a pharmaceutically acceptable carrier).
- the composition can further include a second therapeutic agent.
- the present invention also includes a method of inhibiting abnormal cell growth or treating a proliferative disorder, a destructive bone disorder, an autoimmune disorder, a graft versus host disease, a transplant rejection, an immune deficiency, an inflammatory diseases, an infectious disease, a viral disease, a fibrotic disease, a neurodegenerative disorder, pancreatitis, or a kidney disease in a mammal (e.g. , human), comprising administering to said mammal a therapeutically effective amount of a conjugate represented by Formula (I), a cytotoxic compound represented by Formula (II), or a salt (e.g. , a pharmaceutical acceptable salt) thereof.
- the method described above further comprises administering to said mammal simultaneously, sequentially, or consecutively a second therapeutic (e.g., chemotherapeutic) agent.
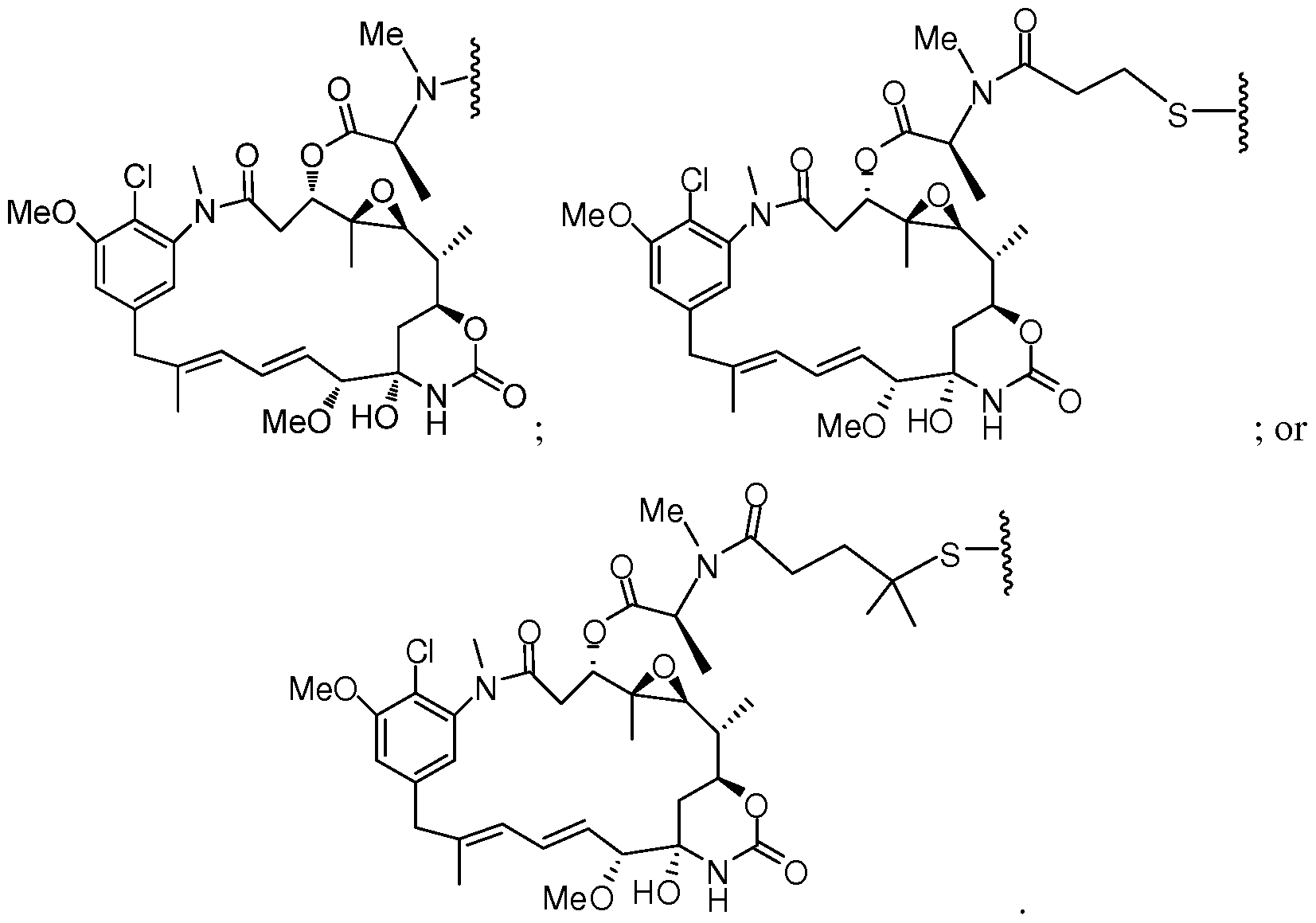
- Figure 1 depicts the structures of certain maytansinoids.
- Figures 2-6 depict the synthetic schemes for preparing cytotoxic compounds each comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates.
- Figures 7 and 8 depict the synthetic schemes for preparing branched linker compounds each with reactive moieties for forming covalent bonds with multiple cytotoxic agents (drugs).
- Figures 9 and 10 depict the synthetic schemes for preparing cytotoxic compounds each comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates.
- Figure 11 depicts the structure of a cytotoxic compound comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates.
- Figure 12 depicts the synthetic scheme for preparing a branched linker compound with reactive moieties for forming covalent bonds with multiple cytotoxic agents (drugs).
- Figures 13-16 depict the synthetic schemes for preparing cytotoxic compounds each comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates.
- Figures 17-23 depict the synthetic schemes for preparing branched linker compounds containing branched scaffolds.
- Figures 24-25 show cell-binding properties of conjugates having branched linkers (i.e., Ab-linker2-(DM4)2 or Ab-linker3-(MayNMA)2 in comparison with naked antibody.
- branched linkers i.e., Ab-linker2-(DM4)2 or Ab-linker3-(MayNMA)2 in comparison with naked antibody.
- Figures 26-28 show the cytotoxicity effects of conjugates having branched linkers (Ab-linker2-(DM4)2 and Ab-linker3-(MayNMA)2 in comparison with those having a non- branched linker (Ab-SPDB-DM4) on COLO205 cells, HCT- 15 cells, and RPMI8226.
- the CBA used in the conjugates is chB38.1.
- SPDB-DM4 4.03D/A, Ab-linker2- (DM4)2 3.1D/A, and Ab-linker3-(MayNMA)2 4.26D/A the cytotoxicity assay was performed in the absence of the competing non-conjugated antibody.
- Figure 29 depicts the synthetic scheme for preparing a cytotoxic compound comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugate.
- Figure 30 depicts the synthetic scheme for preparing a branched linker compound containing branched scaffolds.
- Alkyl refers to a saturated linear or branched-chain monovalent hydrocarbon radical of one to twenty carbon atoms. "Monovalent” means that alkyl has one point of attachment to the remainder of the molecule. Examples of alkyl groups include, but are not limited to, methyl, ethyl, 1 -propyl, 2-propyl, 1 -butyl, 2-methyl-l -propyl, - CH 2 CH(CH 3 ) 2 , 2-butyl, 2-methyl-2-propyl, 1-pentyl, 2-pentyl, 3-pentyl, 2-methyl-2-butyl, 3- methyl-2-butyl, 3-methyl- l -butyl, 2-methyl-l -butyl, 1-hexyl, 2-hexyl, 3-hexyl, 2-methyl-2- pentyl, 3-methyl-2-pentyl, 4-methyl-2-pentyl, 3-methyl-3-pentyl, 2-methyl-3-pentyl, 2-methyl-3-
- Alkylene refers to a saturated linear or branched-chain divalent hydrocarbon radical of one to twenty carbon atoms, examples of which include, but are not limited to, those having the same core structures of the alkyl groups as exemplified above. "Divalent” means that the alkylene has two points of attachment to the remainder of the molecule. Specifically, the alkylene group has one to ten carbon atoms. More specifically, the alkylene group has one to four carbon atoms.
- carrier refers to a monovalent non-aromatic, saturated or partially unsaturated ring having 3 to 12 carbon atoms as a monocyclic ring or 7 to 12 carbon atoms as a bicyclic ring.
- Bicyclic carbocycles having 7 to 12 atoms can be arranged, for example, as a bicyclo [4,5], [5,5], [5,6] or [6,6] system, and bicyclic carbocycles having 9 or 10 ring atoms can be arranged as a bicyclo [5,6] or [6,6] system, or as bridged systems such as bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane and bicyclo[3.2.2]nonane.
- monocyclic carbocycles include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, 1-cyclopent- I-enyl, l-cyclopent-2-enyl, 1-cyclopent- 3-enyl, cyclohexyl, 1-cyclohex-I-enyl, l-cyclohex-2-enyl, l-cyclohex-3-enyl,
- cycloalkylalkyl refers to a cycloalkyl group that is connected to another group by an alkylene group.
- examples of cycloalkylalkyls include, but are not limited to, cyclohexylmethyl, cyclohexylethyl, cyclopentylmethyl, cyclopentylethyl, and the like.
- cycloalkylalkyl is cyclohexylmethyl.
- an integer “between” x and y includes integers x and y unless otherwise specified to the contrary.
- an integer between 1 and 5" can be 1, 2, 3, 4, or 5.
- cyclic alkyl and “cycloalkyl” can be used interchangeably. They refer to a monovalent saturated carbocyclic ring radical. "Monovalent” means that cycloalkyl has one point of attachment to the remainder of the molecule.
- the saturated carbocyclic ring can be monocyclic or bicyclic (fused, bridged, or spiro bicyclic). Specifically, the cycloalkyl is 3 to 7 membered monocyclic ring radical.
- Bicyclic cycloalkyl having 7 to 12 atoms can be arranged, for example, as a bicyclo [4,5], [5,5], [5,6], or [6,6] system, and bicyclic cycloalkyl having 9 or 10 ring atoms can be arranged as a bicyclo [5,6] or [6,6] system, or as bridged systems such as bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane and bicyclo[3.2.2]nonane.
- Examples of monocyclic cycloalkyl groups include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl, cyclodecyl, cycloundecyl, cyclododecyl, and the like. More specifically, the cycloalkyl is cyclohexyl.
- Cycloalkylene refers to a divalent saturated carbocyclic ring radical having 3 to 12 carbon atoms as a monocyclic ring, or 7 to 12 carbon atoms as a bicyclic ring. "Divalent” means that the cycloalkylene has two points of attachment to the remainder of the molecule. Specifically, the cycloalkylene is a 3- to 7-membered monocyclic. Examples of cyclic alkylene groups include, but not limited to, those having the same core structures of the cylcolakyl groups as exemplified above. More specifically, the cycloalkylene group is cyclohexylene.
- alkenyl refers to linear or branched-chain monovalent hydrocarbon radical of two to twenty carbon atoms with at least one carbon-carbon double bond, wherein the alkenyl radical includes radicals having "cis” and “trans” orientations, or by an alternative nomenclature, "E” and “Z” orientations.
- the alkenyl has two to four carbon atoms, also referred to as "C 2 _ 4 alkenyl.”
- Alkenylene refers to linear or branched-chain divalent hydrocarbon radical of two to twenty carbon atoms with at least one carbon-carbon double bond, wherein the alkenyl radical includes radicals having "cis” and “trans” orientations, or by an alternative nomenclature, "E” and “Z” orientations.
- the alkenylene has two to ten carbon atoms, also referred to as "C 2-1 o
- alkenylene More specifically, the alkenylene has two to four carbon atoms, also referred to as "C 2 _ 4 alkenylene.”
- cyclic alkenyl and “cycloalkenyl” can be used interchangeably. They refer to a monovalent carbocyclic ring radical with at least one carbon-carbon double bond, having 3 to 12 carbon atoms as a monocyclic ring, or 7 to 12 carbon atoms as a bicyclic ring (fused, bridged, or spiro bicyclic). "Monovalent” means that cycloalkenyl has one point of attachment to the remainder of the molecule. Specifically, the cycloalkenyl is 3 to 7 membered monocyclic ring radical.
- Bicyclic cycloalkenyl having 7 to 12 atoms can be arranged, for example, as a bicyclo [4,5], [5,5], [5,6], or [6,6] system, and bicyclic cycloalkenyl having 9 or 10 ring atoms can be arranged as a bicyclo [5,6] or [6,6] system, or as bridged systems such as bicyclo[2.2.1]heptene, bicyclo[2.2.2]octene and
- Examples of the monocyclic alkenyl include, but are not limited to, cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, cyclooctenyl, cyclononenyl, cyclodecenyl, cycloundecenyl, cyclododecenyl, and the like. More
- the cycloalkenyl is cyclohexenyl.
- Cycloalkenylene refers to a divalent unsaturated carbocyclic ring radical having 3 to 12 carbon atoms as a monocyclic ring, or 7 to 12 carbon atoms as a bicyclic ring. "Divalent” means that the cycloalkenylene has two points of attachment to the remainder of the molecule. Specifically, the cycloalkenylene is a 3- to 7-membered monocyclic. Examples of cyclic alkenylene include, but not limited to, those having the same core structures of the cylcolakyl groups as exemplified above. More specifically, the cycloalkenylene group is cyclohexenylene.
- Alkynyl refers to linear or branched-chain monovalent hydrocarbon radical of two to twenty carbon atoms with at least one carbon-carbon triple bond.
- “Monovalent” means that alkynyl has one point of attachment to the remainder of the molecule. Examples include, but are not limited to ethynyl, propynyl, 1-butynyl, 2-butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, hexynyl, and the like. Specifically, the alkynyl has two to ten carbon atoms, also referred to as "C 2-1 o alkynyl.” More specifically, the alkynyl has two to four carbon atoms, also referred to as "C 2 -4 alkynyl.”
- Alkynylene refers to an linear or branched-chain divalent hydrocarbon radical of two to twenty carbon atoms with at least one carbon-carbon triple bond, examples of which include, but are not limited to, those having the same core structures of the alkynyl groups as exemplified above.
- “Divalent” means that the alkynylene has two points of attachment to the remainder of the molecule. Specifically, the alkynylene group has one to ten carbon atoms. More specifically, the alkynylene group has one to four carbon atoms.
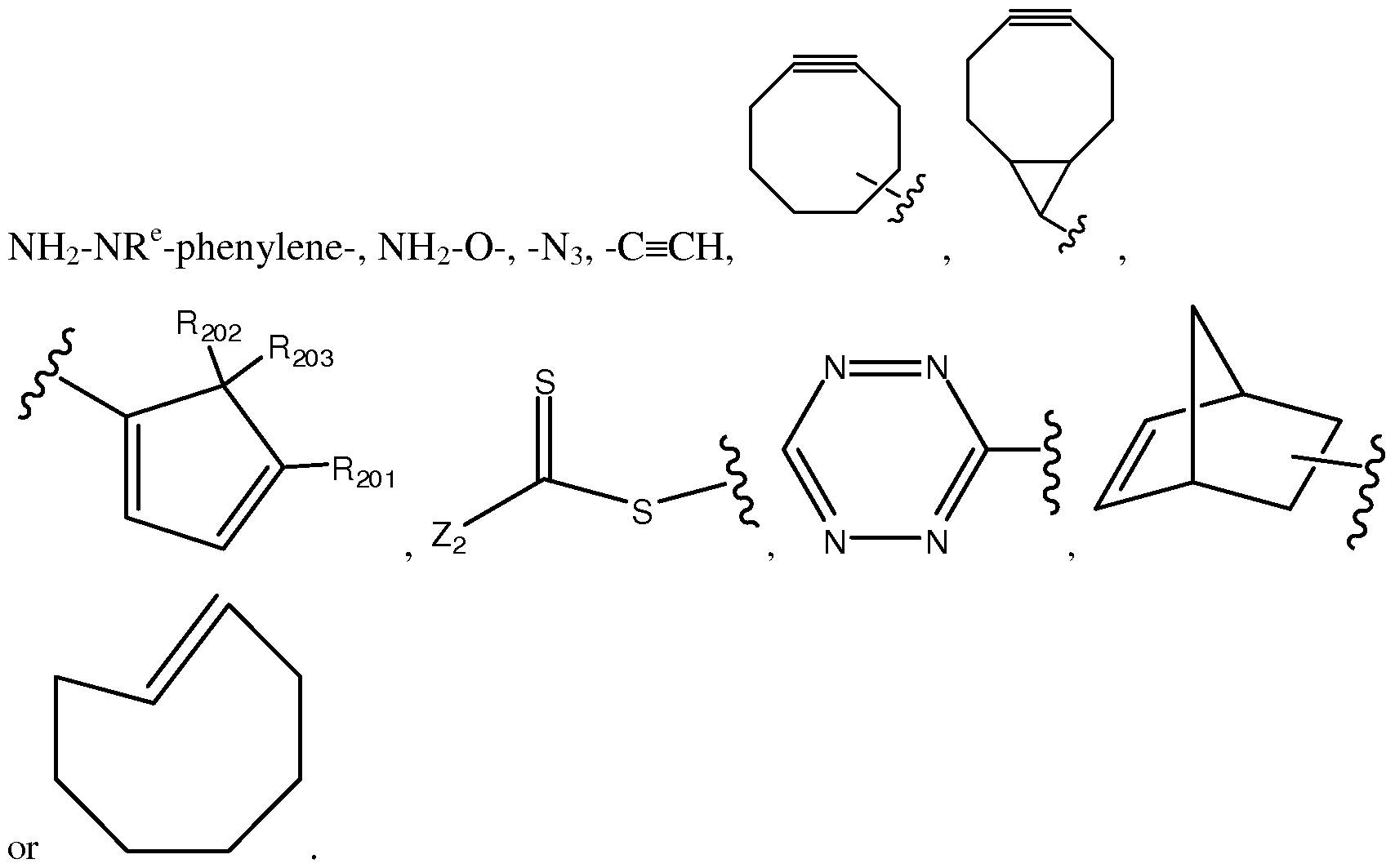
- cyclic alkynyl and “cycloalkynyl” can be used interchangeably. They refer to a monovalent carbocyclic ring radical with at least one carbon-carbon triple bond, having 8 to 12 carbon atoms as a monocyclic ring, or 11 to 17 carbon atoms as a bicyclic ring (fused, bridged, or spiro bicyclic). "Monovalent” means that cycloalkynyl has one point of attachment to the remainder of the molecule. Specifically, the cycloalkynyl is 8-membered monocyclic ring radical.
- Cycloalkyne refers carbocyclic ring having one or more triple bonds. It can be monocyclic, bicyclic or tricyclic; bicyclic and tricyclic can be bridged or fused.
- the carbocyclic ring optionally contains one or more double bonds and/or is optionally fused with one or more aromatic (e.g., phenyl ring) or heteroaromatic rings.
- cycloalkyne examples include, but are not limited to, those described in J. Am. Chem. Soc. (2012) 134:9199-9208; WO 2011/136645, US 2009/0068738, Lang K. et al, J. Am. Chem. Soc.
- cyclooctyne monofluorocyclooctyne, difluorooctyne, DIFO, DIF0 2 , DIFO 3 , bicylo[6.1.0]non-4-yne, benzocyclooctyne, difluorobenzocyclooctyne, dibenzocyclooctyne, DIBO, and those described in Debets, M. F. et al., Acc. Chem. Res., (2011) 44(9):805-815; and Gold B. et al., J. Am. Chem. Soc. (2013) 135(4): 1558-1569.
- cycloalkyne is cyclooctyne.
- Cycloalkynylene refers to a divalent carbocyclic ring radical having at least one carbon-carbon triple bond. "Divalent” means that the cycloalkynylene has two points of attachment to the remainder of the molecule. Specifically, the cycloalkynylene is a 8 to 14-membered monocyclic.
- Heterocycloalkyne refers to a heterocyclic ring having one or more triple bonds. Examples of heterocycloalkyne include, but are not limited to,
- DIBAC dibenzoazacyclooctyne
- BARAC biarylazacyclooctynone
- thiacyclooctyne thiabenzocyclooctyne
- thiacycloheptyne tetramethylthiacycloheptyne
- strained cycloalkene refers to carbocyclic ring having one trans double bond and 7 to 14 ring atoms.
- Examples of strained cycloalkene include, but are not limited to, cyclooctene, norbornene and other cycloalkenes described in Debets, M. F. et al., Acc. Chem. Res. (2011) 44(9):805-815.
- “Strained heterocycloalkene” as used herein refers to a heterocyclic ring having one trans double bonds and 7 to 14 ring atoms selected from carbon and at least one (typically 1 to 4, more typically 1 or 2) heteroatom (e.g., oxygen, nitrogen or sulfur).
- aryl group means an aromatic hydrocarbon ring system having six to fourteen carbon ring atoms.
- aryl may be used interchangeably with the terms “aryl ring” "aromatic ring,” “aryl group” and “aromatic group.”
- Aryl group also includes an aromatic hydrocarbon ring system fused to a non-aromatic carbocyclic ring system, such as a cycloalkyl group. Examples includes phenyl, naphthyl, anthracenyl, 1,2- dihydronaphthyl, 1,2,3,4-tetrahydronaphthyl, fluorenyl, indanyl, indenyl and the like.
- An aryl group is monovalent, i.e., has one point of attachment to the remainder of the molecule.
- a "substituted aryl group” is substituted at any one or more substitutable ring atom, which is a ring carbon atom bonded to a hydrogen.”
- “Arylene” as used herein refers to a divalent aryl group, i.e., an aryl group having two points of attachment to the remainder of the molecule.
- “Divalent” means that the arylene has two points of attachment to the remainder of the molecule. Both aryl and arylene groups are sometime represented herein by "Ar.”
- Arylene is specifically phenylene.
- Heteroaryl (used interchangeably with “heteroaromatic,” “heteroaryl ring,” “heteroaryl group,” “heteroaromatic ring,” and “heteroaromatic group”) refers to aromatic ring systems having five to fourteen ring atoms selected from carbon and at least one
- heteroatoms typically 1 to 4, more typically 1 or 2 heteroatoms (e.g. , oxygen, nitrogen or sulfur).
- Heteroaryl includes monocyclic rings and polycyclic rings (e.g. , bicyclic) in which a monocyclic heteroaromatic ring is fused to one or more other aromatic or heteroaromatic rings.
- “5-14 membered heteroaryl” includes monocyclic, bicyclic or tricyclic ring systems. Heteroaryls are monovalent, meaning that there is one point of attachment to the remainder of the molecule.
- “Monocyclic 5-6 membered heteroaryl” means a monocyclic aromatic ring system having five or six ring atoms selected from carbon and at least one (typically 1 to 3, more typically 1 or 2) heteroatoms (e.g. , oxygen, nitrogen or sulfur).
- Examples of monocyclic 5-6 membered heteroaryl groups include furanyl (e.g., 2-furanyl, 3-furanyl), imidazolyl (e.g., N- imidazolyl, 2-imidazolyl, 4-imidazolyl, 5-imidazolyl), isoxazolyl (e.g., 3-isoxazolyl, 4- isoxazolyl, 5-isoxazolyl), oxadiazolyl (e.g., 2-oxadiazolyl, 5-oxadiazolyl), oxazolyl (e.g., 2- oxazolyl, 4-oxazolyl, 5-oxazolyl), pyrazolyl (e.g.
- pyrazolyl e.g., 1- pyrrolyl, 2-pyrrolyl, 3-pyrrolyl
- pyridyl e.g., 2-pyridyl, 3-pyridyl, 4-pyridyl
- pyrimidinyl e.g., 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl
- pyridazinyl e.g., 3-pyridazinyl
- thiazolyl e.g., 2-thiazolyl, 4-thiazolyl, 5-thiazolyl
- isothiazolyl triazolyl (e.g., 2-triazolyl, 5-triazolyl), tetrazolyl (e.g., tetrazolyl), and thienyl (e.g.
- polycyclic aromatic heteroaryl groups include carbazolyl, benzimidazolyl, benzothienyl, benzofuranyl, isobenzofuranyl, indolyl, benzotriazolyl, benzothiazolyl, benzoxazolyl, quinolinyl, isoquinolinyl, indazolyl, isoindolyl, acridinyl, or benzisoxazolyl.
- a "substituted heteroaryl group” is substituted at any one or more substitutable ring atom, which is a ring carbon or ring nitrogen atom bonded to a hydrogen.
- Heteroarylene refers to a divalent heteroaryl, i.e., a heteroaryl with two points of attachment to the remainder of the molecule.
- heterocycle refers to a saturated or unsaturated non-aromatic 3-12 membered ring radical optionally containing one or more double bonds. It can be monocyclic, bicyclic, or tricyclic; bicyclic and tricyclic can be bridged or fused.
- the heterocycle contains 1 to 4 heteroatoms, which may be the same or different, selected from N, O or S.
- the heterocycle optionally contains one or more double bonds and/or is optionally fused with one or more aromatic (e.g. , phenyl ring) or heteroaromatic rings.
- heterocycle means a radical having from 3-7 atoms (including 1-3 heteroatoms) arranged in a monocyclic ring.
- heterocycle is intended to include all the possible isomeric forms.
- a heterocycle may be a monocycle having 3 to 7 ring members (e.g., 2 to 6 carbon atoms and 1 to 4 heteroatoms selected from N, O, P, and S) or a bicycle having 7 to 10 ring members (e.g., 4 to 9 carbon atoms and 1 to 6 heteroatoms selected from N, O, P, and S), for example: a bicyclo [4,5], [5,5], [5,6], or [6,6] system.
- Heterocycles are described in Paquette, Leo A., Principles of Modern Heterocyclic Chemistry (W. A. Benjamin, New York, 1968), particularly Chapters 1, 3, 4, 6, 7, and 9; The Chemistry of Heterocyclic Compounds, A Series of Monographs (John Wiley & Sons, New York, 1950 to present), in particular Volumes 13, 14, 16, 19, and 28; and /. Am. Chem. Soc. (1960) 82:5566.
- heterocyclic rings include, but are not limited to, aziridinyl, pyrrolidinyl, tetrahydrofuranyl, dihydrofuranyl, tetrahydrothienyl, tetrahydropyranyl, dihydropyranyl, tetrahydrothiopyranyl, tetrahydropyrrolyl, piperidino, morpholino, thiomorpholino, thioxanyl, piperazinyl, homopiperazinyl, azetidinyl, oxetanyl, thietanyl, homopiperidinyl, oxepanyl, thiepanyl, oxazepinyl, diazepinyl, thiazepinyl, 2-pyrrolinyl, 3-pyrrolinyl, indolinyl, isoindolinyl, 2H-pyranyl, 4H-pyranyl, dioxanyl, 1,
- azabicyclo[2.2.2]hexanyl Spiro moieties are also included within the scope of this definition.
- the heterocycle, heteroaryl, or heteroarylene groups may be carbon (carbon-linked) or nitrogen (nitrogen-linked) attached where such is possible.
- carbon bonded heterocycle, heteroaryl or heroarylene groups are bonded at position 2, 3, 4, 5, or 6 of a pyridine, position 3, 4, 5, or 6 of a pyridazine, position 2, 4, 5, or 6 of a pyrimidine, position 2, 3, 5, or 6 of a pyrazine, position 2, 3, 4, or 5 of a furan, tetrahydrofuran, thiophene, pyrrole or tetrahydropyrrole, position 2, 4, or 5 of an oxazole, imidazole or thiazole, position 3, 4, or 5 of an isoxazole, pyrazole, or isothiazole, position 2 or 3 of an aziridine, position 2, 3, or 4 of an azetidine, position 2, 3, 4, 5, 6, 7, or 8 of a quinoline or position
- nitrogen bonded heterocycle, heteroaryl, or heteroarylene groups are bonded at position 1 of an aziridine, azetidine, pyrrole, pyrrolidine, 2-pyrroline, 3-pyrroline, imidazole, imidazolidine, 2-imidazoline, 3-imidazoline, pyrazole, pyrazoline, 2-pyrazoline, 3-pyrazoline, piperidine, piperazine, indole, indoline, IH-indazole, position 2 of a isoindole or isoindoline, position 4 of a morpholine, and position 9 of a carbazole, or O-carboline.
- heteroatoms present in heteroaryl, heteroarylene, or heterocyclcyl can include the oxidized forms such as NO, SO, and S0 2 .
- Halogen refers to F, CI, Br or I.
- a group is described as being “optionally substituted,” the group may be either (1) not substituted, or (2) substituted. If a carbon of a group is described as being optionally substituted with one or more of a list of substituents, one or more of the hydrogen atoms on the carbon (to the extent there are any) may separately and/or together be replaced with an independently selected optional substituent.
- Suitable substituents for an alkyl, alkenyl, alkynyl, cycloalkyl, aryl, heteroaryl, heterocycle, alkylene, alkenylene, alkynylene, cycloalkene, heterocycloalkene, cycloalkyne, heterocycloalkyne, cycloalkylene arylene, and heterarylene are those which do not significantly adversely affect the biological activity of the conjugate.
- the substituent for the optionally substituted alkyl, alkylene, cycloalkylene, arylene, and heteroarylene described above is selected from the group consisting of halogen, -CN, - NR 10 iRio2, -CF 3 , -OR 10 o, aryl, heteroaryl, heterocyclyl, -SR 10 i, -SOR 10 i, -S0 2 Rioi, and -S0 3 M.
- the suitable substituent is selected from the group consisting of - halogen, -OH, -N0 2 , -CN, CM alkyl, -ORioo, NR101R102, -NR101COR102, -SR100, -SO2R101, - SO 2 NR 10 iRi02, -COR101, -OCOR 10 i, and -OCONR 10 iRi 02 , wherein R 100 , R101, and R 102 are each independently -H or C 1-4 alkyl.
- ionizable group refers to a functional group that can be converted to a charged group by protonation with an acid or deprotonation with a base.
- ionizable groups include -S0 3 H, -Z'-S0 3 H, -OP0 3 H 2 , -Z'-OP0 3 H 2 , -P0 3 H 2 , -Z'-P0 3 H 2 , - C0 2 H, -Z'C0 2 H, -NR u Ri 2 , or -Z'-NR u Ri 2 , Rn and R 12 , for each occurrence, are independently H or an optionally substituted alkyl; and Z' includes an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene. In certain embodiments, Z' is alkylene.
- charged substituent refers to a substituent that is either positively or negatively charged.
- the charge in such a substituent is not removable by treatment with a base or an acid and thus permanent.
- the charged substituents include, but not limited to, -N + R 13 R 14 R 15 and -Z'-N + R 13 R 14 R 15 , in which R 1 to R15 are each independently an optionally substituted alkyl; and Z' includes an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene. In certain embodiments, Z' is alkylene.
- Charged substituents may contain a counter ion.
- the counter ion is negative and can be represented by "X " ,” e.g., as -N ⁇ RwR ⁇ X " and -Z'- N + R 13 R 14 R 1 5X " .
- the counter ions for the positively charged substituents are anions
- anions which include, but are not limited to, acetate, benzenesulfonate, benzoate, bicarbonate, bitartrate, bromide, calcium edetate, camsylate, carbonate, chloride, bromide, citrate, dihydrochloride, edetate, edisylate, estolate, esylate, fumarate, glyceptate, gluconate, glutamate, glycollylarsanilate, hexylresorcinate, hydroxynaphthoate, iodide, isethionate, lactate, lactobionate, malate, maleate, mandelate, mesylate, methylsulfate, mucate, napsylate, nitrate, pamoate, pantothenate,
- phosphate/diphospate polygalacturonate, salicylate, stearate, subacetate, succinate, sulfate, tannate, tartrate, teoclate, tosylate, and triethiodide.
- the counter ions for the positively charged substituents are chloride, bromide, sulfate, and phosphate.
- the counter ions for the negatively charged substituents include, but are not limited to, an alkali metal ion (e.g. , sodium and potassium), an alkaline earth metal ion (e.g., calcium and magnesium), aluminum ion, ammonium, protonated trialkyl amines (e.g. , trimethylamine and triethylamine), a tetraalkyl ammonium (e.g. , tetra methyl ammonium, and tetrabutyl ammonium), and a protonated heteroaromatic group (e.g. , pyridine, pyrimidine, triazines, tetrazines).
- an alkali metal ion e.g. , sodium and potassium
- an alkaline earth metal ion e.g., calcium and magnesium
- aluminum ion ion
- ammonium e.g. , ammonium, protonated trialkyl amines (e.g. ,
- the counter ions for the negatively charged substituents are sodium, potassium, lithium, protonated triethyl amine, protonated pyridiene. Most specifically, the counter ions for the negatively charged substituents are sodium and potassium. The counter ions for both the negatively and positively charged substituents may be removed or replaced in subsequent purification steps.
- amino acid as used herein, including the residue represented by variable "XX" described above, refers to naturally occurring amino acids, unnatural amino acids, amino acid analogs, or amino acid mimetics that function in a manner similar to the naturally occurring amino acids.
- Naturally occurring amino acids refers to those twenty L- amino acids encoded by the universal genetic codes and appearing in proteins or peptides, as well as selenocysteine and pyrrolysine that are incorporated into proteins by distinctive biosynthetic mechanisms. They include histidine, alanine, isoleucine, arginine, leucine, asparagine, lysine, aspartic acid, methionine, cysteine, phenylalanine, glutamic acid, threonine, glutamine, tryptophan, glycine, valine, proline, serine, tyrosine, selenocysteine and pyrrolysine.
- naturally occurring amino acids also refers to those produced by the body, but are not encoded by the universal genetic codes, such as ⁇ -alanine, ornithine, and citrulline.
- naturally occurring amino acids further includes those naturally occurring L-amino acids that are later modified (e.g. , via post-translational modification by enzymes) in the body, such as hydroxyproline, ⁇ -carboxyglutamate, and O-phospho serine.
- unnatural amino acids as used herein is intended to include the "D" stereochemical form of the naturally occurring amino acids described above. It is further understood that the term “unnatural amino acids” includes homologues of the natural L- amino acids or their D isomers, and synthetically modified forms of the natural L-amino acids or their D isomers.
- the synthetically modified forms include, but are not limited to, amino acids having side chains shortened or lengthened by up to two carbon atoms, amino acids comprising optionally substituted aryl groups, and amino acids comprised halogenated groups, specifically halogenated alkyl and aryl groups and also N substituted amino acids e.g.
- N-methyl-histidine N-methyl-alanine, N-methyl-isoleucine, N-methyl-arginine, N-methyl- leucine, N-methyl-asparagine, N-methyl-lysine, N-methyl-aspartic acid, N-methyl- methionine, N-methyl-cysteine, N-methyl-phenylalanine, N-methyl-glutamic acid, N-methyl- threonine, N-methyl-glutamine, N-methyl-tryptophan, N-methyl-glycine, N-methyl-valine, N-methyl-proline, N-methyl-serine, N-methyl-tyrosine, N-methyl-selenocysteine, and N- methyl-pyrrolysine, each including an L or D isomer.
- the "unnatural amino acids” includes, for example, glutamic acid 5-methyl ester (Glu(OMe)), including an L or D isomer.
- amino acid analogs refers to compounds that have the same basic chemical structure as a naturally occurring amino acid, i.e., an a carbon that is bound to a hydrogen, a carboxyl group, an amino group, and an R group.
- Such analogs include 3- aminoalanine, 3-dimethylaminoalanine, 2-amino-4-(dimethylamino)butanoic acid, 2,4- diaminobutanoic acid, 2-amino-6-(dimethylamino)hexanoic acid, 2-amino-5- (dimethylamino)pentanoic acid, homoserine, norleucine, cysteine sulfonic acid, cysteine sulfinic acid, methionine sulfoxide, and methionine methyl sulfonium.
- Such analogs may have modified R groups (e.g. , norleucine) or modified peptide backbones, but retain the same basic chemical structure as a naturally occurring amino acid.
- Amino acid analogs also include D isomers of the above-referenced L-isomers.
- amino acid mimetics refers to chemical compounds that have a structure that is different from the general chemical structure of an amino acid, but functions in a manner similar to a naturally occurring amino acid.
- [XX] 2 -io denotes a peptide of 2 to 10 residues.
- Peptides are short polymers of amino acid monomers linked by peptide bonds, the covalent chemical bonds formed between two amino acid monomers when the carboxyl group of N-terminal monomer reacts with the amino group of the C-terminal monomer.
- a preferred peptide length is about two to ten amino acids as described above, although a peptide of longer length may also be used.
- any one of the peptides described herein can be connected to the rest of the molecules in either direction.
- AA1, AA2, and AA3 each represent an amino acid (naturally-occurring, or unnatural)
- AA1-AA2-AA3 in either direction refers to the tripeptide N-AA1-AA2-AA3-C), and the tripeptide N-AA3-AA2-AA1-C).
- peptide sequences are represented conventionally, with the N-terminus to the left and the C-terminus to the right.
- [XX] 1-10 is an amino acid represented by [XX] ⁇ or a peptide represented by [XX] 2-1 o, in which each XX is the residue of an independently selected amino acid selected from the group consisting of: histidine, alanine, isoleucine, arginine, leucine, asparagine, lysine, aspartic acid, methionine, cysteine, phenylalanine, glutamic acid, threonine, glutamine, tryptophan, glycine, valine, proline, serine, tyrosine, selenocysteine, and pyrrolysine, N-methyl-histidine, N-methyl-alanine, N-methyl-isoleucine, N-methyl- arginine, N-methyl-leucine, N-methyl-asparagine, N-methyl-lysine, N-methyl-aspartic acid, N-methyl-methionine,
- Each [XX] can additionally be glutamic acid 5-methyl ester (Glu(OMe)), including an L or D isomer.
- each XX is the residue of an independently selected amino acid selected from glycine or alanine, each independently as an L or D isomer.
- [XX] O is a peptide represented by [XX] 2 -5 (a peptide of 2 to 5 residues).
- the peptide represented by [XX] 2 _ 10 is cleavable by a protease.
- the protease is a protease expressed in tumor tissue.
- the protease is a lysosomal protease.
- the cleavable peptide refers to peptides containing a cleavage recognition sequence of a protease.
- a cleavage recognition sequence for a protease is an amino acid sequence recognized by the protease during proteolytic cleavage.
- Many protease cleavage sites are known in the art, and these and other cleavage sites can be included a linker, a spacer, or a linker moiety. See, e.g., Matayoshi et al., Science 247:954 (1990); Dunn et al., Meth.
- the peptide sequence is chosen based on its ability to be cleaved by a tumor-associated protease, e.g., a protease that is found on the surface of a cancerous cell or extracellularly in the vicinity of tumor cells.
- a tumor-associated protease e.g., a protease that is found on the surface of a cancerous cell or extracellularly in the vicinity of tumor cells.
- proteases include thimet oligopeptidase (TOP), CDIO (neprilysin), a matrix metalloprotease (such as MMP2 or MMP9), a type II transmembrane serine protease (such as Hepsin, testisin, TMPRSS4 or matriptase/MT-SPl), legumain and enzymes described in the following reference: Current Topics in Developmental Biology: Cell Surface Proteases, vol. 54 Zucker S. 2003, Boston, MA.
- TOP thimet oligopeptidase
- CDIO neprilysin
- MMP9 matrix metalloprotease
- MMP9 type II transmembrane serine protease
- TMPRSS4 type II transmembrane serine protease
- legumain and enzymes described in the following reference Current Topics in Developmental Biology: Cell Surface Proteases, vol. 54 Zucker S. 2003, Boston, MA.
- the peptide sequence is chosen based on its ability to be cleaved by a lysosomal protease, which include cathepsins B, C, D, H, L and S, and furin. Specifically, the peptide sequence is capable of being cleaved by an appropriate isolated protease in vitro, which can be tested using in vitro protease cleavage assays known in the art.
- the peptide is selected from the group consisting of Val-Cit, Val-Lys, Phe-Lys, Lys-Lys, Ala-Lys, Phe-Cit, Leu-Cit, Lle-Cit, Trp, Cit, Phe-Ala, Phe-N 9 - tosyl-Arg, Phe-N 9 -nitro-Arg, Phe-Phe-Lys, D-Phe-Phe-Lys, Gly-Phe-Lys, Leu-Ala-Leu, Ile- Ala-Leu, Val-Ala-Val, Ala-Leu- Ala-Leu, ⁇ -Ala-Leu- Ala-Leu, Gly-Phe-Leu-Gly, Val-Arg, Arg-Val, Arg-Arg, Val-D-Cit, Val-D-Lys, Val-D-Arg, D- Val-Cit, D-Val-Lys, D-Val-Lys, D-
- the peptide is selected from the group consisting of Gly-Gly-Gly, Ala- Ala- Ala, D- Ala- Ala- Ala, Ala-D- Ala- Ala, and Ala-Val-Ala.
- the peptide is Gly-Gly-Ala, Val-Ala, Glu-Ala, or Glu(OMe)-Ala.
- any of the peptide sequences herein above may be in either direction, as defined above.
- An amino acid or peptide can be attached to or be present in a linker or in a spacer, or be attached to a cell binding agent through the terminal amine or terminal carboxylic acid of the amino acid or peptide.
- the amino acid can also be attached to a linker or a spacer, or a cell-binding agent through a side chain reactive group, including, but not restricted to, the thiol group of cysteine, the epsilon amine of lysine, and the side chain hydroxyls of serine or threonine.
- spacer includes a chemical moiety interposed between any two chemical groups.
- one end of the spacer is linked to a cell-bind agent (e.g., an antibody, such as a human or humanized monoclonal antibody, or an antigen-binding portion or fragment thereof), or a reactive functional group that can form a covalent bond with a cell-binding agent.
- a cell-bind agent e.g., an antibody, such as a human or humanized monoclonal antibody, or an antigen-binding portion or fragment thereof
- a reactive functional group that can form a covalent bond with a cell-binding agent.
- one end of the spacer is linked to a cytotoxic drug (e.g., a maytansinoid, such as DM1 or DM4), or a reactive functional group that can form a covalent bond with a cytotoxic drug.
- one end of the spacer is linked to a branched scaffold.
- the spacer is interposed between (1) a cell-binding agent, or a reactive functional group that can form a covalent bond with a cell-binding agent; and (2) a branched scaffold.
- the spacer is interposed between (1) a cytotoxic drug, or a reactive functional group that can form a covalent bond with a cytotoxic drug; and (2) a branched scaffold.
- a spacer may be attached to a reactive functional group at one end to form a linker moiety that can further react with a cell-binding agent or a cytotoxic drug.
- the spacer creates a desired distance between the two chemical groups to, for example, avoid stereo hindrance or to promote molecular flexibility.
- the presence of the spacer does not hinder, inhibit, or otherwise negatively affect the function of the flanking chemical groups (e.g., the ability of the cell- binding agent to bind a target molecule on a cell, or the cytotoxicity of the cytotoxic drug).
- the spacer confers additional beneficial characteristics, such as enhanced potency, solubility, serum stability, and/or efficacy, to the immunoconjugate or linker compound comprising the spacer.
- the spacer may comprise one or more amino acid residues (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more residues), which may or may not be resistant to protease or peptidases (such as intracellular / lysosomal peptidase) cleavage.
- the spacer may comprise one or more repeats of polyethylene glycol (PEG) units -(CH 2 -CH 2 -0)-, such as 1-1000 PEG units, 1-500 PEG units, 1-24 PEG units, or 2-8 PEG units (2, 4, 6, or 8 PEG units). Exemplary spacers hi and L 2 are described in more details below.
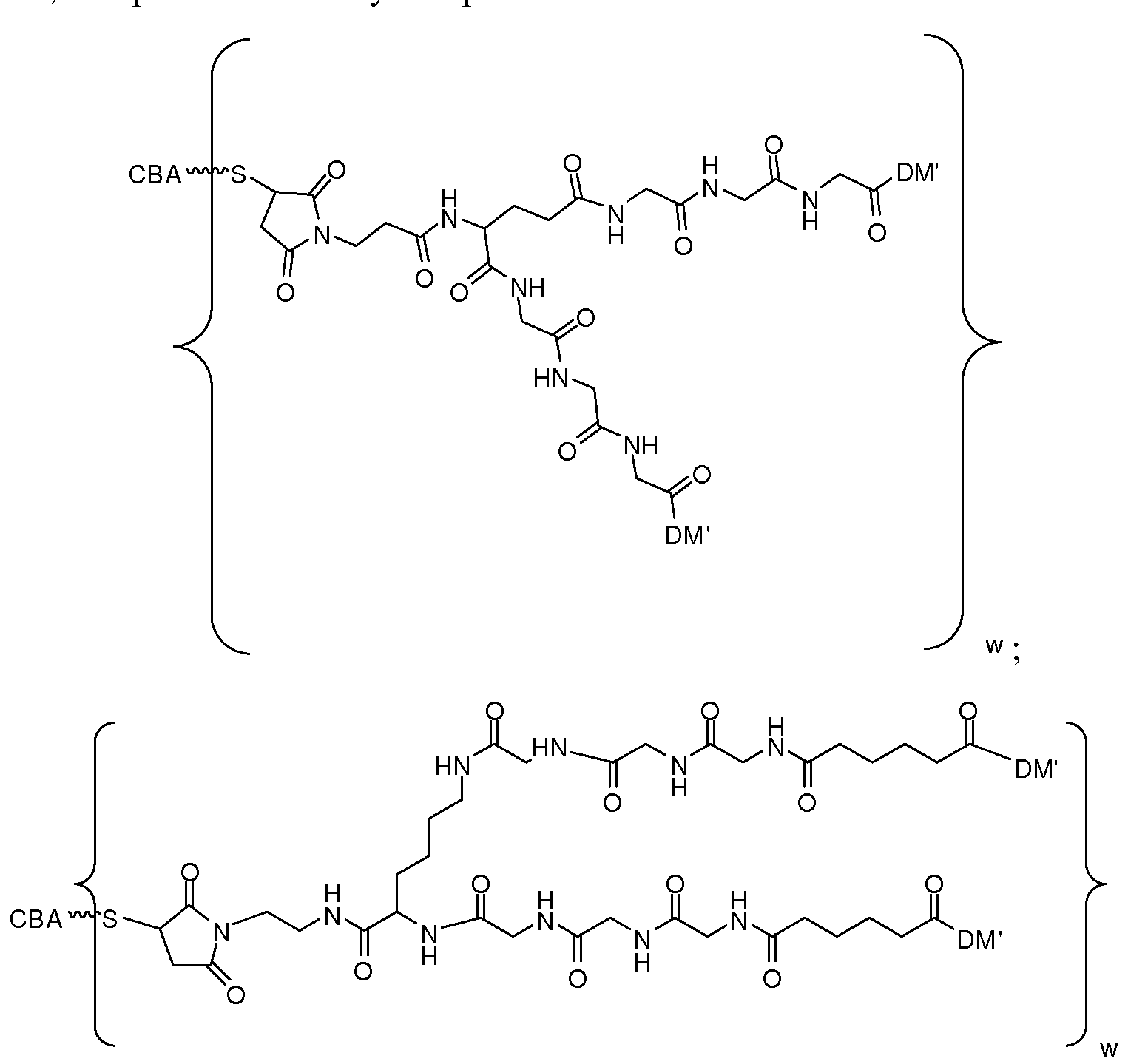
- branched scaffold includes a chemical moiety linked to three or more spacers.
- a branched scaffold allows two or more drugs to be attached to the cell binding agent.
- Exemplary branched scaffolds may be derived from an amino acid with a side chain comprising an amino group (such as Lys) or a carboxyl group (such as a Glu or an Asp), or a peptide comprising two or more such amino acids (e.g., Lys-Lys dimer etc.).
- Each amino acid may be independently D- or L-amino acids.
- Chemically modified amino acids or analogs thereof having similar structures may also give rise to branches scaffolds.
- one cell-binding agent and two or more cytotoxic drugs may be linked to the branched scaffold, each independently through a spacer (same or different).
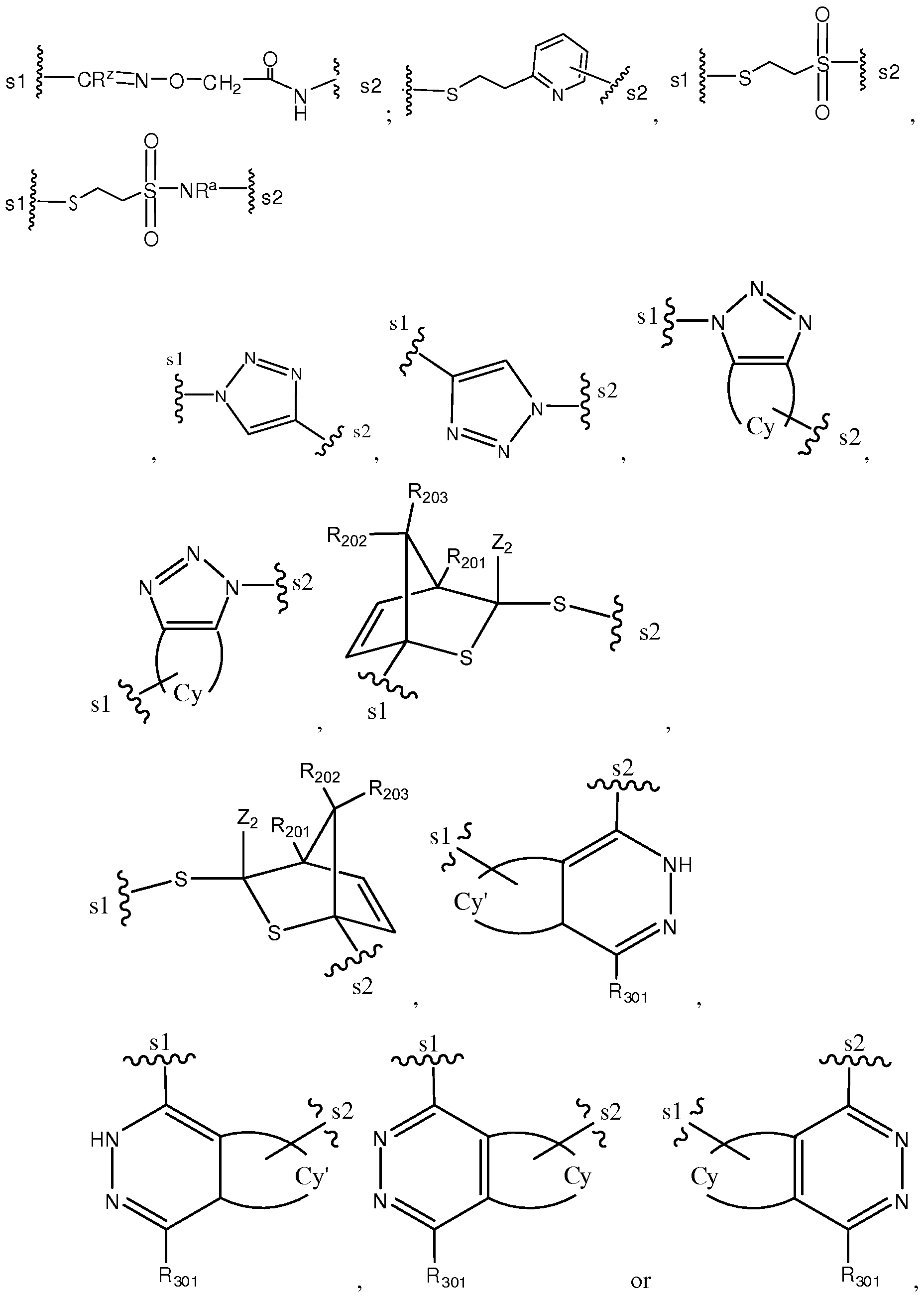
- residue of a functional group is a moiety remaining after a reaction with another functional group.
- a residue of thiol group can form disulfide bond after reacting with another thiol group or thioether after reacting with an electrophilic group, such as maleimide.
- reactive ester refers to an ester group having a leaving group that is readily displaced by an amine group.
- Examples of a reactive ester include, but are not limited to, N-hydroxysuccinimide ester, N-hydroxysulfosuccinimide ester, nitrophenyl (e.g., 2 or 4-nitrophenyl) ester, dinitrophenyl (e.g., 2,4-dinitrophenyl) ester, sulfo- tetraflurophenyl (e.g., 4-sulfo-2,3,5,6-tetrafluorophenyl)ester and pentafluorophenyl ester.
- N-hydroxysuccinimide ester N-hydroxysulfosuccinimide ester
- nitrophenyl e.g., 2 or 4-nitrophenyl
- dinitrophenyl e.g., 2,4-dinitrophenyl
- sulfo- tetraflurophenyl e.g., 4-sulfo-2,3,5,6-tetrafluoroph
- DARPin and "(designed) ankyrin repeat protein” are used interchangeably to refer to certain genetically engineered antibody mimetic proteins typically exhibiting preferential (sometimes specific) target binding.
- the target may be protein, carbohydrate, or other chemical entities, and the binding affinity can be quite high.
- the DARPins may be derived from natural ankyrin repeat-containing proteins, and specifically consist of at least three, usually four or five ankyrin repeat motifs (typically about 33 residues in each ankyrin repeat motif) of these proteins.
- a DARPin contains about four- or five-repeats, and may have a molecular mass of about 14 or 18 kDa, respectively.
- Libraries of DARPins with randomized potential target interaction residues with diversities of over 10 12 variants can be generated at the DNA level, for use in selecting DARPins that bind desired targets (e.g., acting as receptor agonists or antagonists, inverse agonists, enzyme inhibitors, or simple target protein binders) with picomolar affinity and specificity, using a variety of technologies such as ribosome display or signal recognition particle (SRP) phage display.
- desired targets e.g., acting as receptor agonists or antagonists, inverse agonists, enzyme inhibitors, or simple target protein binders
- SRP signal recognition particle
- AVIBODYTM cell binding agents include a family of proteins as cell binding agents that specifically bind desired targets. As is well known, antibodies bind such desired targets through “Target Binding Regions” (TBRs) or Fv domains. AVIBODYTM CBA typically contains two, three, or four TBRs more commonly known as Dia-, Tria- and Tetra-bodies. These TBRs / Fv domains are linked together by fusing the Fv V-domains together in a "head to tail” orientation, forming stable, specific, and highly customizable multimeric antibody-like proteins as AVIBODYTM CBA. See, for example, U.S. Publication Nos. 2008/0152586 and 2012/0171115 for details, the entire teachings of which are incorporated herein by reference.
- cell binding agent refers to a compound that can bind a cell (e.g., on a cell-surface ligand) or bind a ligand associated with or proximate to the cell, either in a specific or non-specific manner. In certain embodiments, binding to the cell or a ligand on or near the cell is specific.
- the cell-binding agent may be of any kind presently known, or that become known and includes peptides and non-peptides.
- the cell-binding agents are proteins or polypeptides, or compounds comprising proteins or polypeptides, including antibody and non-antibody proteins or polypeptides.
- the cell-binding agents e.g., proteins or polypeptides
- the cell-binding agents comprise one or more Cys residues.
- the side chain -SH group of the Cys residues may be intact, or may be in a disulfide bond that can be reduced.
- reduction of the disulfide bond(s) does not significantly negatively impact the cell-binding function of the proteins or polypeptides (e.g., in the case of antibody or antigen-binding portion thereof, reduction of the disulfide bonds does not substantially increase the dissociation of light chains / heavy chains).
- a cell-binding agent e.g., a protein such as an antibody, or polypeptide
- a cell-binding agent may be a part of a modified cell-binding agent, which is linked to a spacer connected to a branched scaffold, which branched scaffold is capable of being connected to two or more cytotoxic drugs, each through a reactive functional group attached to a spacer linked to the branched scaffold.
- An exemplary such modified cell- binding agent is represented in Formula (IV).
- heterocycloalkene, dithioester, or diene see, for example, Vu Hong et al, Bioconjugate Chem. (2010) 21(10): 1912-1916; Glassner, M. et al., J. Am. Chem. Soc. (2012) 134:7274- 7277; Hansell, C. F. et al, J. Am. Chem. Soc. (2011) 133: 13828-13831; Neal K. Devaraj, Synlett (2012) 23(15):2147-2152; Chenoweth, K. et al, Organic & Biomolecular Chemistry (2009) (24):5255; Jewett, J.C. et al, J. Am. Chem. Soc.
- the reactive functional group can be introduced into the modified cell-binding agent through any chemical or enzymatic method known in the art. See, for example, Davis L.K. et al., /. Am. Chem. Soc. (2012) 134: 10317- 10320; Boeggeman E, et al., Bioconjug Chem.
- Cell-binding agent can also be peptides derived from phage display (see, for example, Wang et al, Proc. Natl. Acad. Sci. USA (2011) 108(17):6909-6914) or peptide library techniques (see, for example, Dane et al., Mol. Cancer. Ther. (2009) 8(5): 1312-1318).
- one or more reactive functional groups that are capable of reacting with the cytotoxic drug can be introduced into the modified cell-binding agent (e.g., Formula (IV)) by any methods known in the art.
- a terminal amine group on the modified cell-binding agent can be converted to a carbonyl group through transamination reaction (see, for example, US 2010/0099649; Angew. Chem. Int. Ed., 45(32):5307 (2006); Chem. Biol., 2(4):247 (2007); /. Am. Chem. Soc, 130(35): 11762, 2008).
- the cell-binding agent may be linked to a spacer through a free Cys residue, which can be engineered into the cell-binding agent if necessary (i.e., cysteine residues having a free -SH group that can react with a reactive functional group attached to a spacer) according to any methods known in the art (see, for example, US 7,521,541).
- cysteine residues having a free -SH group that can react with a reactive functional group attached to a spacer i.e., cysteine residues having a free -SH group that can react with a reactive functional group attached to a spacer
- thiol groups thiol groups (-SH) can be generated by controlled reduction of interchain disulfides of antibodies, followed by treatment with a maleimido group as the reactive functional group, as described in US patent 7,659,241.
- Thiol groups can also be introduced into the cell-binding agent (e.g., antibodies) by reaction with a crosslinking agent such as 2-iminothiolane (see for example Goff and Carroll, Bioconjugate Chem. (1990) 1(6):381— 386) followed by reaction with a maleimido group to form a modified cell-binding agent.
- a crosslinking agent such as 2-iminothiolane (see for example Goff and Carroll, Bioconjugate Chem. (1990) 1(6):381— 386) followed by reaction with a maleimido group to form a modified cell-binding agent.
- All these methods for introducing reactive functional groups are applicable of being used for cell-binding agents that are not antibodies, which, for example, include Centyrin, DARPin, Avibody, adnectin or antibody fragment, such as minibodies, diabodies, tribodies, tetrabodies, nanobodies, probodies, domain bodies or unibodies.
- one or more reactive functional groups e.g., a cysteine having a free thiol group
- a cysteine having a free thiol group can be introduced according to methods described in US 2010/0255056, US 2010/0216708 and US 2011/0274623.
- the cell-binding agent is a DARPin and it can be prepared according to methods described in US Publication Nos. 2004/0132028, 2009/0082274,
- DARPin comprises one or more cysteine residues at specific positions that do not interfere with antigen binding. Such cysteine residue can react with the reactive functional groups attached to a spacer.
- Avibodies having one or more cysteine residues can be prepared according to methods described in US 2008/0139791 and US 2012/0171115.
- the Cys side chain -SH groups may react with a reactive functional group described above covalently linked to a spacer connected to the branched scaffold, which can in turn be linked to two or more cytotoxic compounds through additional reactive functional groups attached to spacers, thus conjugating the cell-binding agents to the cytotoxic compounds to yield the conjugates of the invention (e.g., conjugates of Formula (I)).
- Each protein-based cell-binding agents may contain multiple Cys side chain -SH groups, each available for linking the cell-binding agent to a spacer.
- Examples of the cell binding agents include an antibody, a single chain antibody, an antibody fragment that specifically binds to the target cell, a monoclonal antibody, a single chain monoclonal antibody, a monoclonal antibody fragment that specifically binds to a target cell, a chimeric antibody, a chimeric antibody fragment that specifically binds to the target cell, a bispecific antibody, a domain antibody, a domain antibody fragment that specifically binds to the target cell, an interferon, a lymphokine (e.g., IL-2, IL-3, IL-4, and IL-6), a hormone (e.g., insulin, thyrotropin releasing hormone, melanocyte- stimulating hormone, and a steroid hormone (e.g., androgen and estrogen)), a vitamin (e.g., folate), a growth factor (e.g., EGF, TGF-alpha, FGF, VEGF), a colony stimulating factor, a nutrient- transport molecule (
- Centyrin a protein scaffold based on a consensus sequence of fibronectin type III (FN3) repeats; see U.S. Patent Publication Nos.
- an Ankyrin Repeat Protein e.g., a designed ankyrin repeat protein, known as DARPin; see U.S. Patent Publication Nos. 2004/0132028, 2009/0082274, 2011/0118146, and 2011/0224100, incorporated herein by reference, and also see C. Zahnd et al., Cancer Res. (2010) 70: 1595- 1605; Zahnd et al, J. Biol. Chem. (2006) 281(46):35167-35175; and Binz, H.K., Amstutz, P. & Pluckthun, A., Nature Biotechnology (2005) 23: 1257-1268, incorporated herein by reference), an ankyrin-like repeats protein or synthetic peptide (see e.g., U.S. Patent
- WO 2007/062466 incorporated herein by reference
- an Adnectin a fibronectin domain scaffold protein; see US Patent Publication Nos. 2007/0082365; 2008/0139791, incorporated herein by reference
- Avibody including diabodies, triabodies, and tetrabodies; see U.S. Publication Nos. 2008/0152586 and 2012/0171115
- other cell-binding molecules or substances see U.S. Publication Nos. 2008/0152586 and 2012/0171115
- the cell-binding agent is an antibody, a single chain antibody, an antibody fragment that specifically binds to the target cell, a monoclonal antibody, a single chain monoclonal antibody, a monoclonal antibody fragment that specifically binds to a target cell, a chimeric antibody, a chimeric antibody fragment that specifically binds to the target cell, a domain antibody, a domain antibody fragment that specifically binds to the target cell, a lymphokine, a hormone, a vitamin, a growth factor, a colony stimulating factor, or a nutrient-transport molecule.
- the cell-binding agent is a monoclonal antibody, a single chain monoclonal antibody, or a monoclonal antibody fragment that specifically binds to a target cell.
- the cell-binding agent is a bispecific antibody, an ankyrin repeat protein, a Centyrin, or an Avibody.
- Antibody fragment and "antigen-binding portion or fragment” are used interchangeably here to refer to Fab, Fab', and F(ab') 2 , Fv, minibodies, diabodies, tribodies, tetrabodies, nanobodies, probodies, domain bodies, unibodies, and the like (Parham, J.
- the cell-binding agent is a minibody, a diabody, a tribody, a tetrabody, a nanobody, a probody, a domain body, or an unibody.
- Monoclonal antibody techniques allow for the production of extremely specific cell- binding agents in the form of specific monoclonal antibodies.
- Particularly well known in the art are techniques for creating monoclonal antibodies produced by immunizing mice, rats, hamsters or any other mammal with the antigen of interest such as the intact target cell, antigens isolated from the target cell, whole virus, attenuated whole virus, and viral proteins such as viral coat proteins.
- Sensitized human cells can also be used.
- Another method of creating monoclonal antibodies is the use of phage libraries of scFv (single chain variable region), specifically human scFv ⁇ see e.g., Griffiths et al, U.S. Patent Nos.
- resurfaced antibodies disclosed in U.S. Patent No. 5,639,641 may also be used, as may chimeric antibodies and humanized antibodies.
- Selection of the appropriate cell-binding agent is a matter of choice that depends upon the particular cell population that is to be targeted, but in general human monoclonal antibodies are preferred if an appropriate one is available.
- the monoclonal antibody MY9 is a murine IgGi antibody that binds specifically to the CD33 Antigen (J.D. Griffin et ah, Leukemia Res. (1984) 8:521) and can be used if the target cells express CD33 as in the disease of acute myelogenous leukemia (AML).
- the cell-binding agent is a resurfaced antibody, a resurfaced single chain antibody, or a resurfaced antibody fragment.
- the cell-binding agent is a humanized antibody, a humanized single chain antibody, or a humanized antibody fragment.
- the humanized antibody is huMy9-6 or another related antibody, which is described in U.S. Pat. Nos. 7,342,110 and 7,557, 189.
- the humanized antibody is an anti-folate receptor antibody described in U.S. Provisional Application Nos. 61/307,797, 61/346,595, and 61/413,172 and U.S. Application No. 13/033,723 (published as US
- the cell-binding agent is an antigen -binding portion of a monoclonal antibody, sharing sequences critical for antigen-binding with an antibody disclosed herein, such as huMy9-6 or its related antibodies described in U.S. Pat. Nos.
- These derivative antibodies may have substantially the same or identical (1) light chain and/or heavy chain CDR3 regions; (2) light chain and/or heavy chain CDR1, CDR2, and CDR3 regions; or (3) light chain and/or heavy chain regions, compared to an antibody described herein. Sequences within these regions may contain conservative amino acid substitutions, including substitutions within the CDR regions. Specifically, there is no more than 1, 2, 3, 4, or 5 conservative substitutions. In an alternative, the derivative antibodies have a light chain region and/or a heavy chain region that is at least about 90%, 95%, 99% or 100% identical to an antibody described herein.
- These derivative antibodies may have substantially the same binding specificity and/or affinity to the target antigen compared to an antibody described herein. Specifically, the K d and/or R values of the derivative antibodies are within 10-fold (either higher or lower), 5-fold (either higher or lower), 3-fold (either higher or lower), or 2-fold (either higher or lower) of an antibody described herein.
- These derivative antibodies may be fully human antibodies, or humanized antibodies, or chimeric antibodies.
- the derivative antibodies may be produced according to any art-recognized methods.
- Specific exemplary antigens or ligands include renin; a growth hormone (e.g.
- human growth hormone and bovine growth hormone a growth hormone releasing factor; a parathyroid hormone; a thyroid stimulating hormone; a lipoprotein; alpha- 1- antitrypsin; insulin A-chain; insulin B-chain; proinsulin; a follicle stimulating hormone; calcitonin; a luteinizing hormone; glucagon; a clotting factor (e.g.
- factor vmc factor IX, tissue factor, and von Willebrands factor
- an anti-clotting factor e.g., Protein C
- an atrial natriuretic factor e.g., a lung surfactant
- a plasminogen activator e.g., a urokinase, a human urine or tissue-type plasminogen activator
- bombesin e.g., a thrombin
- hemopoietic growth factor e.g., tumor necrosis factor- alpha and -beta
- an enkephalinase RANTES (i.e., the regulated on activation normally T-cell expressed and secreted)
- human macrophage inflammatory protein- 1 -alpha a serum albumin (human serum albumin); Muellerian-inhibiting substance; relaxin A-chain; relaxin B-chain; prorelaxin; a mouse gonadotropin-associated peptide; a microbial protein (
- inhibin activin; a vascular endothelial growth factor; protein A or D; a rheumatoid factor; a neurotrophic factor(e.g. , bone-derived neurotrophic factor, neurotrophin-3, -4, -5, or -6), a nerve growth factor (e.g.
- NGF- ⁇ a platelet-derived growth factor
- a fibroblast growth factor e.g., aFGF and bFGF
- fibroblast growth factor receptor 2 an epidermal growth factor
- a transforming growth factor e.g., TGF-alpha, TGF- ⁇ , TGF- 2, TGF- 3, TGF- 4, and TGF- ⁇ 5
- insulin-like growth factor-I and -II des(l-3)-IGF-I (brain IGF-I)
- an insulin-like growth factor binding protein melanotransferrin; EpCAM; GD3; FLT3; PSMA; PSCA; MUC1 ; MUC16; STEAP; CEA; TENB2; an EphA receptor; an EphB receptor; a folate receptor; FOLR1 ; mesothelin; cripto; an alpha v beta 6 ; integrins; VEGF; VEGFR; EGFR; transferrin receptor; IRTAl ;
- CD81, CD103, CD105, CD134, CD137, CD138, and CD152 one or more tumor- associated antigens or cell-surface receptors (see US Publication No. 20080171040 or US Publication No. 20080305044, incorporated in their entirety by reference); erythropoietin; an
- osteoinductive factor an immunotoxin; a bone morphogenetic protein; an interferon (e.g. , interferon-alpha, -beta, and -gamma); a colony stimulating factor (e.g., M-CSF, GM-CSF, and G-CSF); interleukins (e.g., IL- 1 to IL- 10); a superoxide dismutase; a T-cell receptor; a surface membrane protein; a decay accelerating factor; a viral antigen s,(e.g.
- a portion of the HIV envelope a transport protein, a homing receptor; an addressin; a regulatory protein; an integrin (e.g., CDl la, CDl lb, CDl lc, CD18, an ICAM, VLA-4, and VCAM;) a tumor associated antigen (e.g. , HER2, HER3 and HER4 receptor); endoglin; c-Met; c-kit; 1GF1R; PSGR; NGEP; PSMA; PSCA; TMEFF2; LGR5; B7H4; and fragments of any of the above- listed polypeptides.
- an integrin e.g., CDl la, CDl lb, CDl lc, CD18, an ICAM, VLA-4, and VCAM
- a tumor associated antigen e.g. , HER2, HER3 and HER4 receptor
- GM-CSF a ligand / growth factor which binds to myeloid cells
- IL-2 which binds to activated T-cells can be used for prevention of transplant graft rejection, for therapy and prevention of graft-versus-host disease, and for treatment of acute T-cell leukemia.
- MSH which binds to melanocytes, can be used for the treatment of melanoma, as can antibodies directed towards melanomas.
- Folic acid can be used to target the folate receptor expressed on ovarian and other tumors.
- Epidermal growth factor can be used to target squamous cancers, such as lung and head and neck.
- Somatostatin can be used to target neuroblastomas and other tumor types.
- Estrogen or estrogen analogues
- Androgen or androgen analogues
- testes can be used to target testes.
- salt refers to organic or inorganic salts of a compound of the invention. Specifically, a salt is a pharmaceutically acceptable salt. Other non- pharmaceutically acceptable salts are also included in the present invention.
- the salts include salts, formed by reacting a compound of the invention, which comprises a basic group, with an inorganic acid or organic acid (such as a carboxylic acid), and salts, formed by reacting a compound of the invention, which comprises an acidic group, with an inorganic base or organic base (such as an amine).
- Exemplary salts include those pharmaceutically acceptable salts described immediately below.
- Exemplary salts include, but are not limited, to sulfate, citrate, acetate, oxalate, chloride, bromide, iodide, nitrate, bisulfate, phosphate, acid phosphate, isonicotinate, lactate, salicylate, acid citrate, tartrate, oleate, tannate, pantothenate, bitartrate, ascorbate, succinate, maleate, gentisinate, fumarate, gluconate, glucuronate, saccharate, formate, benzoate, glutamate, methanesulfonate "mesylate,” ethanesulfonate, benzenesulfonate, p-toluenesulfonate, pamoate (i.e.
- a pharmaceutically acceptable salt may involve the inclusion of another molecule such as an acetate ion, a succinate ion or other counter ion.
- the counter ion may be any organic or inorganic moiety that stabilizes the charge on the parent compound.
- a pharmaceutically acceptable salt may have more than one charged atom in its structure. Instances where multiple charged atoms are part of the pharmaceutically acceptable salt can have multiple counter ions. Hence, a pharmaceutically acceptable salt can have one or more charged atoms and/or one or more counter ion.
- desired salts may be prepared by any suitable method available in the art, for example, treatment of the free base with an inorganic acid, such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, methanesulfonic acid, phosphoric acid and the like, or with an organic acid, such as acetic acid, maleic acid, succinic acid, mandelic acid, fumaric acid, malonic acid, pyruvic acid, oxalic acid, glycolic acid, salicylic acid, a pyranosidyl acid, such as glucuronic acid or galacturonic acid, an alpha hydroxy acid, such as citric acid or tartaric acid, an amino acid, such as aspartic acid or glutamic acid, an aromatic acid, such as benzoic acid or cinnamic acid, a sulfonic acid, such as p-toluen
- an inorganic acid such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid
- desired salts may be prepared by any suitable method, for example, treatment of the free acid with an inorganic, such as an alkali metal hydroxide or alkaline earth metal hydroxide, organic base, such as an amine (primary, secondary or tertiary), or the like.
- an inorganic such as an alkali metal hydroxide or alkaline earth metal hydroxide
- organic base such as an amine (primary, secondary or tertiary), or the like.
- suitable salts include, but are not limited to, organic salts derived from amino acids, such as glycine and arginine, ammonia, primary, secondary, and tertiary amines, and cyclic amines, such as piperidine, morpholine and piperazine, and inorganic salts derived from sodium, calcium, potassium, magnesium, manganese, iron, copper, zinc, aluminum and lithium.
- amino acids such as glycine and arginine
- ammonia such as glycine and arginine
- primary, secondary, and tertiary amines such as piperidine, morpholine and piperazine
- inorganic salts derived from sodium, calcium, potassium, magnesium, manganese, iron, copper, zinc, aluminum and lithium.
- abnormal cell growth and “proliferative disorder” are used interchangeably in this application.
- abnormal cell growth refers to cell growth that is independent of normal regulatory
- tumor cells tumors
- mutated tyrosine kinase or overexpression of a receptor tyrosine kinase tumor cells
- benign and malignant cells of other proliferative diseases in which aberrant tyrosine kinase activation occurs tumor cells that proliferate by expressing a mutated tyrosine kinase or overexpression of a receptor tyrosine kinase
- benign and malignant cells of other proliferative diseases in which aberrant tyrosine kinase activation occurs any tumors that proliferate by receptor tyrosine kinases
- any tumors that proliferate by aberrant serine/threonine kinase activation and (5) benign and malignant cells of other proliferative diseases in which aberrant serine/threonine kinase activation occurs.
- cancer refers to or describe the physiological condition in mammals that is typically characterized by unregulated cell growth.
- a “tumor” comprises one or more cancerous cells, and/or benign or pre-cancerous cells.
- cancer include, but are not limited to, carcinoma, lymphoma, blastoma, sarcoma, and leukemia or lymphoid malignancies. More particular examples of such cancers include skin cancer (e.g., melanoma), Merkel cell carcinoma, squamous cell cancer (e.g. , epithelial squamous cell cancer), lung cancer (e.g.
- small-cell lung cancer non-small cell lung cancer, adenocarcinoma of the lung, and squamous carcinoma of the lung
- cancer of the peritoneum hepatocellular cancer
- gastric or stomach cancer e.g. , gastrointestinal cancer
- pancreatic cancer cervical cancer, ovarian cancer, liver cancer, bladder cancer, hepatoma, breast cancer, colon cancer, rectal cancer, colorectal cancer, endometrial or uterine carcinoma, salivary gland carcinoma, kidney or renal cancer, prostate cancer, testicular cancer, vulval cancer, thyroid cancer, hepatic carcinoma, anal carcinoma, penile carcinoma, acute leukemia, head and neck cancer, brain cancer (e.g., glioblastoma and neuroblastoma), cancers of lymphatic organs and hematological malignancy including Leukemia (Acute lymphoblastic leukemia (ALL), Acute myelogenous leukemia (AML), Chronic lymphocytic leukemia (CLL), Chronic myelog
- cytotoxic compound is referred to as “D” or “DM” interchangeably.
- chemotherapeutic agent or "cytotoxic drug” includes is a chemical compound useful in the treatment of cancer.
- chemotherapeutic agents include Erlotinib (TARCEVA®, Genentech/OSI Pharm.), Bortezomib (VELCADE®, Millennium Pharm.), Fulvestrant (FASLODEX®, AstraZeneca), Sutent (SU11248, Pfizer), Letrozole (FEMARA®, Novartis), Imatinib mesylate (GLEEVEC®, Novartis), PTK787/ZK 222584 (Novartis), Oxaliplatin (Eloxatin®, Sanofi), 5-FU (5-fluorouracil), Leucovorin, Rapamycin (Sirolimus, RAPAMUNE®, Wyeth), Lapatinib (TYKERB®, GSK572016, Glaxo Smith Kline), Lonafarnib (SCH 66336), Sorafenib (BAY43
- acetogenins especially bullatacin and bullatacinone
- a camptothecin including the synthetic analog topotecan
- bryostatin callystatin
- CC-1065 including its adozelesin, carzelesin and bizelesin synthetic analogs
- cryptophycins particularly cryptophycin 1 and cryptophycin 8
- dolastatin duocarmycin (including the synthetic analogs, KW-2189 and CB1-TM1);
- pancratistatin a sarcodictyin
- spongistatin nitrogen mustards such as chlorambucil, chlornaphazine, chlorophosphamide, estramustine, ifosfamide,
- calicheamicin omegall Angew Chem. Intl. Ed. Engl. (1994) 33: 183-186
- dynemicin including dynemicin A; bisphosphonates, such as clodronate; an esperamicin; as well as neocarzino statin chromophore and related chromoprotein enediyne antibiotic chromophores), aclacinomysins, actinomycin, authramycin, azaserine, bleomycins, cactinomycin, carabicin, caminomycin, carzinophilin, chromomycinis, dactinomycin, daunorubicin, detorubicin, 6- diazo-5-oxo-L-norleucine, ADRIAMYCIN ® (doxorubicin), morpholino-doxorubicin, cyanomorpholino-doxorubicin, 2-pyrrolino-doxorubic
- methotrexate, pteropterin, trimetrexate purine analogs such as fludarabine, 6- mercaptopurine, thiamniprine, thioguanine; pyrimidine analogs such as ancitabine, azacitidine, 6-azauridine, carmofur, cytarabine, dideoxyuridine, doxifluridine, enocitabine, floxuridine; androgens such as calusterone, dromostanolone propionate, epitiostanol, mepitiostane, testolactone; anti-adrenals such as aminoglutethimide, mitotane, trilostane; folic acid replenisher such as frolinic acid; aceglatone; aldophosphamide glycoside;
- aminolevulinic acid aminolevulinic acid; eniluracil; amsacrine; bestrabucil; bisantrene; edatraxate; defofamine; demecolcine; diaziquone; elformithine; elliptinium acetate; an epothilone; etoglucid; gallium nitrate; hydroxyurea; lentinan; lonidainine; maytansinoids such as maytansine and ansamitocins; mitoguazone; mitoxantrone; mopidanmol; nitraerine; pentostatin; phenamet; pirarubicin; losoxantrone; podophyllinic acid; 2-ethylhydrazide; procarbazine; PSK ® polysaccharide complex (JHS Natural Products, Eugene, Oreg.); razoxane; rhizoxin;
- vindesine dacarbazine; mannomustine; mitobronitol; mitolactol; pipobroman; gacytosine; arabinoside ("Ara-C”); cyclophosphamide; thiotepa; taxoids, e.g., TAXOL ® (paclitaxel; Bristol-Myers Squibb Oncology, Princeton, N.J.), ABRAXANE® (Cremophor-free), albumin-engineered nanoparticle formulations of paclitaxel (American Pharmaceutical Partners, Schaumberg, 111.), and TAXOTERE ® (doxetaxel; Rhone-Poulenc Rorer, Antony, France); chloranmbucil; GEMZAR ® (gemcitabine); 6-thioguanine; mercaptopurine;
- methotrexate platinum analogs such as cisplatin and carboplatin; vinblastine; etoposide (VP- 16); ifosfamide; mitoxantrone; vincristine; NAVELBINE ® (vinorelbine); novantrone;
- teniposide edatrexate; daunomycin; aminopterin; capecitabine (XELODA ® ); ibandronate; CPT-11 ; topoisomerase inhibitor RFS 2000; difluoromethylomithine (DMFO); retinoids such as retinoic acid; and pharmaceutically acceptable salts, acids and derivatives of any of the above.
- chemotherapeutic agent or "cytotoxic drug” are: (i) anti-hormonal agents that act to regulate or inhibit hormone action on tumors such as anti-estrogens and selective estrogen receptor modulators (SERMs), including, for example, tamoxifen (including NOLVADEX ® ; tamoxifen citrate), raloxifene, droloxifene, 4- hydroxytamoxifen, trioxifene, keoxifene, LY 117018, onapristone, and FARESTON ® (toremifine citrate); (ii) aromatase inhibitors that inhibit the enzyme aromatase, which regulates estrogen production in the adrenal glands, such as, for example, 4(5)-imidazoles, aminoglutethimide, MEGASE ® (megestrol acetate), AROMASIN ® (exemestane; Pfizer), formestanie, fadrozole, RIVISOR
- SERMs selective estrogen receptor modul
- ARIMIDEX ® anastrozole; AstraZeneca
- anti-androgens such as flutamide, nilutamide, bicalutamide, leuprolide, and goserelin; as well as troxacitabine (a 1,3-dioxolane nucleoside cytosine analog)
- protein kinase inhibitors e.g., IL-12 kinase inhibitors
- lipid kinase inhibitors lipid kinase inhibitors
- antisense oligonucleotides particularly those which inhibit expression of genes in signaling pathways implicated in aberrant cell proliferation, such as, for example, PKC-alpha, Ralf and H-Ras
- ribozymes such as VEGF expression inhibitors (e.g., ANGIOZYME ® ) and HER2 expression inhibitors
- vaccines such as gene therapy vaccines, for example,
- anti-angiogenic agents include MMP-2 (matrix - metalloproteinase 2) inhibitors, MMP-9 (matrix-metalloproteinase 9) inhibitors, COX-II (cyclooxygenase II) inhibitors, and VEGF receptor tyrosine kinase inhibitors.
- VEGF receptor tyrosine kinase inhibitors include 4-(4-bromo-2-fluoroanilino)- 6-methoxy-7-(l-methylpiperidin-4-ylmethoxy)quinazoline (ZD6474; Example 2 within WO 01/32651), 4-(4-fluoro-2-methylindol-5-yloxy)-6-methoxy-7-(3-pyrrolidin- l-ylpropoxy)- quinazoline (AZD2171 ; Example 240 within WO 00/47212), vatalanib (PTK787; WO 98/35985) and SU11248 (sunitinib; WO 01/60814), and compounds such as those disclosed in PCT Publication Nos. WO 97/22596, WO 97/30035, WO 97/32856, and WO 98/13354).
- chemotherapeutic agents / cytotoxic drugs that can be used in combination with the present compounds include inhibitors of PI3K (phosphoinositide-3 kinase), such as those reported in Yaguchi et al (2006) Jour, of the Nat. Cancer Inst.
- PI3K phosphoinositide-3 kinase
- PI3K inhibitors include SF-1126 (PI3K inhibitor, Semafore Pharmaceuticals), BEZ-235 (PI3K inhibitor, Novartis), XL-147 (PI3K inhibitor, Exelixis, Inc.).
- Chemotherapeutic agents / cytotoxic drugs may also include any of the generic drugs or biosimilars of the brand-name drugs referenced herein, or improvements thereof, including improved formulations, delivery means (sustained release, bioadhesive coating, targeted delivery etc.), and dosage forms.
- viral infection refers to the invasion of a host organism' s bodily tissues by disease-causing viruses.
- examples of the viral infections include CMV infection, HIV infection and AIDS.
- parasite infection refers to the invasion of a host organism's bodily tissues by disease-causing parasites.
- examples of the parasite infections include giardiasis, amoebiasis, and schistosomiasis.
- phrases "pharmaceutically acceptable” indicates that the substance or composition must be compatible chemically and/or toxicologically, with the other ingredients comprising a formulation, and/or the mammal being treated therewith.
- terapéuticaally effective amount means that amount of active compound or conjugate that elicits the desired biological response in a subject. Such response includes alleviation of the symptoms of the disease or disorder being treated, prevention, inhibition or a delay in the recurrence of symptom of the disease or of the disease itself, an increase in the longevity of the subject compared with the absence of the treatment, or prevention, inhibition or delay in the progression of symptom of the disease or of the disease itself. Determination of the effective amount is well within the capability of those skilled in the art, especially in light of the detailed disclosure provided herein. Toxicity and therapeutic efficacy of compound I can be determined by standard pharmaceutical procedures in cell cultures and in experimental animals.
- the effective amount of compound or conjugate of the present invention or other therapeutic agent to be administered to a subject will depend on the stage, category and status of the multiple myeloma and characteristics of the subject, such as general health, age, sex, body weight and drug tolerance.
- the effective amount of compound or conjugate of the present invention or other therapeutic agent to be administered will also depend on administration route and dosage form. Dosage amount and interval can be adjusted individually to provide plasma levels of the active compound that are sufficient to maintain desired therapeutic effects.
- hi is represented by the following formula:
- H sl is the site covalently linked to the CBA
- s2 is the site covalently linked to the group U;
- Ar is an optionally substituted arylene or an optionally substituted heteroarylene
- Cy is an optionally substituted carbocyclic or heterocyclic ring
- R201, R202 and R203 each are independently H or an optionally substituted alkyl;
- Z 2 is pyridyl or
- R 2 04 and R 2 os are each independently optionally substituted alkyl
- R301 is H or optionally substituted alkyl
- Cy' is an optionally substituted carbocyclic or heterocyclic ring
- Ai is absent, an optionally substituted alkylene, an optionally substituted
- cycloalkylene an optionally substituted cycloalkylalkylene, -(CH 2 -CH 2 -0) pl -(CR b R c ) p r- or -(CR b R C ) PR -(0-CH 2 -CH 2 ) pl -,
- pi' is an integer from 0 to 10;
- R 9 is H or an optionally substituted alkyl
- R a , R b , R c and R e are independently H or an optionally substituted alkyl.
- pi is 1 to 24. In another alternative, pi is 1 to 8. In yet another alternative, pi is 2, 4, 6, or 8. The remainder of the variables in Formula (I) are as described in the first embodiment.
- Ri and R 2 for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
- the remainder of the variables in Formula (I) or each of the above formulas representing hi are as defined in the first embodiment or its first or second specific embodiment.
- h is represented by the following formula:
- Ri and R 2 are each independently H or an alkyl
- n is an integer from 1 to 5;
- R 9 is H or an alkyl.
- L 2 is represented by the following formula:
- a 2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]j.io;
- [XX] for each occurrence, is independently an amino acid residue
- R b and R c are independently H or an optionally substituted alkyl (more specifically H);
- R b and R c are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group
- p2 is an integer from 1 to 1000 (specifically 1 to 14, 1 to 8);
- p2' is an integer from 1 to 10 (specifically 1 to 5);
- p2" is an integer from 1 to 10 (specifically 1 to 5).
- R b and R c are H; p2 is 1-14; and p2'is 1 to 5; and in another alternative, R b and R c are H; p2 is 1-8; and p2'is 1 to 5.
- the remainder of the variables in Formula (I) are as defined in the first embodiment or its first, second, third, fourth, fifth, or sixth specific embodiment.
- L 2 is represented by the following formula:
- R 12 , R 13 , and R 14 are each independently H, an optionally substituted alkyl
- Q is H, a charged substituent or an ionizable group
- r is an integer from 0 to 10;
- s5 is the site covalently linked to the group U;
- s6 is the site covalently linked to the group D.
- the conjugate is represented by the following formula, or a pharmaceutically acceptable salt thereof:
- Q is i) H; ii) -S0 3 H, -Z'-S0 3 H, -OP0 3 H 2 , -Z'- OP0 3 H 2 , -P0 3 H 2 , -Z'-P0 3 H 2 , -C0 2 H, -Z'-C0 2 H, -NR 10 iRi 02 ,-Z'-NR 10 iRio2, or a
- Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R 102 , are each independently H or an optionally substituted alkyl; R 103 to R 10 5 are each independently an optionally substituted alkyl; and X " is a pharmaceutically acceptable anion.
- the remainder of the variables in Formula (I) or each of the formulas designated in the eighth or ninth embodiment above are as defined in the first embodiment or its eighth or ninth specific embodiment.
- R 12 is H; and Q is H, SO 3 H or a pharmaceutically acceptable salt thereof.
- the remainder of the variables in Formula (I) or each of the formulas designated in the eighth or ninth embodiment above are as defined in the first embodiment or its eighth, ninth or tenth specific embodiment.
- R b and R c are both H; and p2' is an integer from 1 to 5.
- the remainder of the variables in Formula (I) or each of the formulas designated in the seventh, eighth, or ninth specific embodiment are as defined in the first embodiment or its seventh, eighth, ninth, tenth, or eleventh specific embodiment.
- R 13 and R 14 are both H; and r is 1 to 5.
- the remainder of the variables in Formula (I) or each of the formulas designated in the eighth, or ninth embodiment are as defined in the first embodiment or its eighth, ninth, tenth, eleventh, or twelfth specific embodiment.
- the conjugate is represented by one of the following formulas, or a pharmaceutically acceptable salt thereof:
- D is a maytansinoid in the conjugate in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth specific embodiment.
- D in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth specific embodiment is represented by:
- R, R' and R" for each occurrence, are independently H or an optionally substituted alkyl; and Y is an optionally substituted alkylene, an optionally substituted alkenylene, or an optionally substituted alkynlene.
- R 15 to R 18 are independently H or an optionally substituted alkyl, an optionally substituted alkenyl, an optionally substituted cycloalkyl, an optionally substituted heterocyclyl, an optionally substituted aryl or an optionally substituted heteroaryl;
- r is an integer between 0 to 15.
- R 15 to R ⁇ are independently H or an optionally substituted alkyl.
- R 15 to R ⁇ are all H and r is 1.
- R15 and R 16 are H, r is 2, and R 17 and R 18 are both methyl. .
- D in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, fourteenth, fifteenth, sixteenth, or seventeenth specific embodiment is represented by the followin formula:
- the conjugate is represented by the following formula, or a pharmaceutically acceptable salt thereof:
- DM' is represented by the following formula:
- w is specifically an integer between 1 and 6 or alternatively, between 1 and 4.
- the present invention also provides a cytotoxic compound represented by Formula (II) below:
- hi is represented by the following formula:
- optionally substituted arylene or an optionally substituted heteroarylene is an optionally substituted cycloalkyne or an optionally substituted heterocycloalkyne
- R 201 , R202 and R203 each are independently H or an optionally substituted alkyl
- Z 2 is pyridyl or
- R 2 o 4 and R205 are each independently optionally substituted alkyl, is an optionally substituted strained cycloalkene or an optionally substituted strained heterocycloalkene;
- X' is a halogen
- X is -OH or a carboxylic acid activating group (specifically COX” is a reactive ester);
- Z is H or -SR d ;
- R a , R b , R c and R e are independently H or an optionally substituted alkyl
- R d is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
- Ai is absent, an optionally substituted alkylene, an optionally substituted
- cycloalkylene an optionally substituted cycloalkylalkylene, -(CH 2 -CH 2 -0) pl -(CR b R c ) pl '- or -(CR b R c ) p r-(0-CH 2 -CH 2 ) pl -;
- pi is an integer from 1 to 1000 (specifically, 1 to 24, more specifically 1 to 8 (e.g, 2, 4, 6 or 8));
- pi ' is an integer from 0 to 10;
- R9 is H or an optionally substituted alkyl.
- COX is a reactive ester and pi is 1 to 24. In another alternative, COX" is a reactive ester and pi is 1 to 8. In yet another alternative, COX" is a reactive ester and pi is 2, 4, 6, or 8. Alternatively, The remainder of the variables in Formula (II) are as described in the second embodiment.
- R and R 2 for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
- R and R 2 for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
- the remainder of the variables in Formula (II) are as defined in the second embodiment or its first or second specific embodiment.
- J CB is -COX" or a maleimide
- R and R 2 for each occurrence, are each independently H or an alkyl
- m is an integer from 1 to 5
- R 9 is H or an alkyl.
- R and R 2 are both H; and R 9 is H.
- the remainder of the variables in Formula (II) or each of the above formulas representing are as defined in the second embodiment or its third or fourth specific embodiment.
- L 2 is represented by the following formula:
- a 2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX] 1-10 ;
- [XX] for each occurrence, is independently an amino acid residue
- R b and R c are independently H or an optionally substituted alkyl (Specifically H);
- R b and R c are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group
- p2 is an integer from 1 to 1000 (specifically 1 to 14, 1 to 8);
- p2' is an integer from 1 to 10 (specifically 1 to 5);
- p2" is an integer from 1 to 10 (specifically 1 to 5).
- R b and R c are H; p2 is 1-14; and p2'is 1 to 5; and in another alternative, R b and R c are H; p2 is 1-8; and p2'is 1 to 5.
- the remainder of the variables in Formula (II) are as defined in the second embodiment or its first, second, third, fourth, fifth, or sixth specific embodiment.
- L 2 is represented by the following formula:
- R 12 , Ri 3 , and R 14 are each independently H, an optionally substituted alkyl
- Q is H, a charged substituent or an ionizable group
- r is an integer from 0 to 10;
- s5 is the site covalently linked to the group U;
- s6 is the site covalently linked to the group D.
- the cytotoxic compound is represented by the following formula, or a salt thereof:
- Q is i) H; ii) -S0 3 H, -Z'-S0 3 , -OP0 3 H 2 , -Z'-OP0 3 H 2 , -P0 3 H 2 , -Z'-P0 3 H 2 , -C0 2 H, -Z'-C0 2 H, -NR 10 iRio 2 ,-Z'-NR 10 iRio 2 , or a pharmaceutically acceptable salt thereof; or iii) or -Z'-N + R 1 o 3 Rio4RiosX ; Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R 102 , are each independently H or an optionally substituted alkyl; Rio 3 to Rios are each independently an optionally substituted alkyl; and X " is a pharmaceutically acceptable anion.
- R 12 is H; and Q is H, S0 3 H or a salt (e.g., pharmaceutically acceptable salt) thereof.
- the remainder of the variables in Formula (II) or each of the formulas designated in the eighth or ninth embodiment above are as defined in the second embodiment or its eighth, ninth, or tenth specific embodiment.
- R b and R c are both H; and p2' is an integer from 1 to 5.
- the remainder of the variables in Formula (II) or each of the formulas designated in the seventh, eighth, or ninth specific embodiment are as defined in the second embodiment or its seventh, eighth, ninth, tenth, or eleventh specific embodiment.
- R 13 and R 14 are both H; and r is 1 to 5.
- the remainder of the variables in Formula (II) or each of the formulas designated in the eighth, or ninth embodiment are as defined in the second embodiment or its eighth, ninth, tenth, eleventh, or twelfth specific embodiment.
- the cytotoxic compound is represented by one of the following formulas or a salt thereof:
- D is a maytansinoid in the cytotoxic compound in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth specific embodiment.
- D in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth specific embodiment is represented by:
- R, R' and R" for each occurrence, are independently H or an optionally substituted alkyl
- Y is an optionally substituted alkylene, an optionally substituted alkenylene, or an optionally substituted alkynlene.
- R 15 to R 18 are independently H or an optionally substituted alkyl, an optionally substituted alkenyl, an optionally substituted cycloalkyl, an optionally substituted heterocyclyl, an optionally substituted aryl or an optionally substituted heteroaryl;
- r is an integer between 0 to 15.
- R15 to R 18 are independently H or an optionally substituted alkyl.
- R 15 to R 18 are all H and r is 1.
- R 15 and R 16 are H, r is 2, and R 17 and R 18 are both methyl. .
- D in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, fourteenth, fifteenth, sixteenth, or seventeenth specific embodiment is represented by the following formula:
- the cytotoxic compound is represented by the following formula, or a salt thereof:
- the present invention further provides a linker compound represented by Formula (III) below:
- linker compound sixteen specific embodiments for the linker compound are further described below in this linker compound section.
- R 201 , R202 and R203 each are independently H or an optionally substituted alkyl;
- Z 2 is pyridyl or
- R 2 o 4 and R205 are each independently optionally substituted alkyl
- a nd is an optionally substituted strained cycloalkene or an optionally substituted strained heterocycloalkene
- X' is a halogen
- X is -OH or a carboxylic acid activating group (specifically COX” is a reactive ester);
- Z is H or -SR d ;
- R a , R b , R c and R e are independently H or an optionally substituted alkyl
- R d is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
- Ai is absent, an optionally substituted alkylene, an optionally substituted
- cycloalkylene an optionally substituted cycloalkylalkylene, -(CH 2 -CH 2 -0) pl -(CR b R c ) pl - or -(CR b R c ) p r-(0-CH 2 -CH 2 ) pl -;
- pi is an integer from 1 to 1000 (specifically, 1 to 24, more specifically 1 to 8 (e.g, 2, 4, 6 or 8));
- pi ' is an integer from 0 to 10;
- R9 is H or an optionally substituted alkyl.
- COX is a reactive ester and pi is 1 to 24. In another alternative, COX" is a reactive ester and pi is 1 to 8. In yet another alternative, COX" is a reactive ester and pi is 2, 4, 6, or 8. The remainder of the variables in Formula (III) are as described in the third embodiment.
- h ⁇ is represented by the following formula:
- R and R 2 for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
- R and R 2 for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
- J CB is -COX" or a maleimide
- R and R 2 for each occurrence, are each independently H or an alkyl
- R9 is H or an alkyl
- m is an integer from 1 to 5.
- the remainder of the variables in Formula (III) or each of the formulas designated in the third specific embodiment are as defined in the third embodiment or its first, second, or third specific embodiment.
- R and R 2 are both H; and R 9 is H.
- the remainder of the variables in Formula (III) or each of the above formulas representing hi are as defined in the third embodiment or its third or fourth specific embodiment.
- h ⁇ is represented by the following formula:
- L 2 ' is represented by the following formula:
- a 2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX] 1-10 ;
- X" is -OH or a carboxylic acid activating group
- Z is H or -SR d ;
- R d is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
- [XX] for each occurrence, is independently an amino acid residue
- R b and R c are independently H or an optionally substituted alkyl (specifically H);
- R b and R c are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group
- p2 is an integer from 1 to 1000 (specifically 1 to 14, 1 to 8);
- p2' is an integer from 1 to 10 (specifically 1 to 5);
- p2" is an integer from 1 to 10 (specifically 1 to 5).
- R b and R c are H; p2 is 1-14; and p2'is 1 to 5; and in another alternative, R b and R c are H; p2 is 1-8; and p2'is 1 to 5.
- the remainder of the variables in Formula (III) are as defined in the third embodiment or its first, second, third, fourth, fifth, or sixth specific embodiment.
- L 2 ' is represented by the following formula:
- R 12 , R 13 , and R 14 are each independently H, an optionally substituted alkyl
- Q is H, a charged substituent or an ionizable group
- r is an integer from 0 to 10;
- s5 is the site covalently linked to the group U.
- R d is a pyridyl or nitropyridyl.
- the compound is represented by the following formula, or a salt thereof:
- Q is i) H; ii) -SO 3 H, -Z'-S0 3 H, -OP0 3 H 2 , -Z'- OP0 3 H 2 , -P0 3 H 2 , -Z'-P0 3 H 2 , -C0 2 H, -Z'-C0 2 H, -NRioiRio 2 ,-Z'-NRioiRio2, or a
- R 12 is H; and Q is H, S0 3 H or a salt (e.g., pharmaceutically acceptable salt) thereof.
- the remainder of the variables in Formula (III) or each of the formulas designated in the eighth or tenth embodiment above are as defined in the third embodiment or its eighth, ninth, tenth, eleventh specific embodiment.
- R b and R c are both H; and p2' is an integer from 1 to 5.
- the remainder of the variables in Formula (III) or each of the formulas designated in the seventh, eighth, or tenth specific embodiment are as defined in the third embodiment or its seventh, eighth, ninth, tenth, eleventh or twelfth specific embodiment.
- R 13 and R 14 are both H; and r is 1 to 5.
- the remainder of the variables in Formula (III) or each of the formulas designated in the eighth, or ninth embodiment are as defined in the third embodiment or its eighth, ninth, tenth, eleventh, twelfth, or thirteenth specific embodiment.
- the present invention further provides a modified cell binding agent represented by Formula (IV) below:
- hi is represented by the following formula:
- si is the site covalently linked to the CBA
- s2 is the site covalently linked to the group U;
- Ar is an optionally substituted arylene or an optionally substituted heteroarylene
- Cy is an optionally substituted carbocyclic or heterocyclic ring
- R201, R202 and R203 each are independently H or an optionally substituted alkyl
- Z 2 is pyridyl or ;
- R204 and R205 are each independently optionally substituted alkyl
- R301 is H or optionally substituted alkyl
- Cy' is an optionally substituted carbocyclic or heterocyclic ring
- Ai is absent, an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH 2 -CH 2 -0) p1 -(CR b R c ) p1 -(CR b R c ) p r-(0-CH 2 -CH 2 ) pl -,
- pi ' is an integer from 0 to 10;
- R 9 is H or an optionally substituted alkyl; and R ⁇ R b , R c , R e and R z , for each occurrence, are independently H or an optionally substituted alkyl.
- pi is 1 to 24. In another alternative, pi is 1 to 8. In yet another alternative, pi is 2, 4, 6, or 8. The remainder of the variables in Formula (IV) are as described in the fourth embodiment.
- hi is represented by the following formula:
- R and R 2 for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
- R and R 2 for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
- h is represented by the following formula:
- Ri and R 2 are each independently H or an alkyl
- n is an integer from 1 to 5;
- R 9 is H or an alkyl.
- Ri and R 2 are both H; and R 9 is H.
- the remainder of the variables in Formula (IV) or each of the formulas designated in the third or fourth specific embodiment are as defined in the fourth embodiment or its third or fourth specific embodiment.
- Li is represented by the following formula:
- L 2 ' is represented by the following formula:
- a 2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]no;
- X" is -OH or a carboxylic acid activating group
- Z is H or -SR d ;
- R d is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
- [XX] for each occurrence, is independently an amino acid residue
- R b and R c are independently H or an optionally substituted alkyl (specifically H);
- R b and R c are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group
- p2 is an integer from 1 to 1000 (specifically 1 to 14, 1 to 8);
- p2' is an integer from 1 to 10 (specifically 1 to 5);
- p2" is an integer from 1 to 10 (specifically 1 to 5).
- R b and R c are H; p2 is 1-14; and p2'is 1 to 5; and in another alternative, R b and R c are H; p2 is 1-8; and p2'is 1 to 5.
- the remainder of the variables in Formula (IV) are as defined in the fourth embodiment or its first, second, third, fourth, fifth, or sixth specific embodiment.
- L 2 ' is represented by the following formula:
- R 12 , Ri3, and R 14 are each independently H, an optionally substituted alkyl
- Q is H, a charged substituent or an ionizable group
- r is an integer from 0 to 10.
- R d is a pyridyl or nitropyridyl.
- the modified cell binding agent is represented by the following formula or a salt thereof:
- Q is i) H; ii) -S0 3 H, -Z'-S0 3 H, -OP0 3 H 2 , -Z'- OP0 3 H 2 , -P0 3 H 2 , -Z'-P0 3 H 2 , -C0 2 H, -Z'-C0 2 H, -NRioiRio 2 ,-Z'-NRioiRio2, or a
- Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R 102 , are each independently H or an optionally substituted alkyl; Rio 3 to Rios are each independently an optionally substituted alkyl; and X " is a pharmaceutically acceptable anion.
- the remainder of the variables in Formula (IV) or each of the formulas designated in the eighth or tenth embodiment above are as defined in the fourth embodiment or its eighth, ninth, or tenth specific embodiment.
- R 12 is H; and Q is H, S0 3 H or a salt (e.g., pharmaceutically acceptable salt) thereof.
- the remainder of the variables in Formula (IV) or each of the formulas designated in the eighth or tenth embodiment above are as defined in the fourth embodiment or its eighth, ninth, tenth, eleventh specific embodiment.
- R b and R c are both H; and p2' is an integer from 1 to 5.
- the remainder of the variables in Formula (IV) or each of the formulas designated in the seventh, eighth, or tenth specific embodiment are as defined in the fourth embodiment or its seventh, eighth, ninth, tenth, eleventh or twelfth specific embodiment.
- R 13 and R 14 are both H; and r is 1 to 5.
- the remainder of the variables in Formula (IV) or each of the formulas designated in the eighth, or ninth embodiment are as defined in the fourth embodiment or its eighth, ninth, tenth, eleventh, twelfth, or thirteenth specific embodiment.
- modified cell binding agent is represented by one of the following formulas, or a salt thereof:
- w is specifically an integer between 1 and 6 or alternatively, between 1 and 4.
- w is specifically an integer between 1 and 6 or alternatively, between 1 and 4.
- R3, R 4 , R 5 , R 6 , R7, Rs, Rio and Rn, for each occurrence, are independently H or an optionally substituted alkyl;
- n, n' , and n" are independently an integer from 1 to 10;
- R 501 is H or an optionally substituted alkyl
- s3 is the site covalently linked the group hi;
- s4 is the site covalently linked to the group L 2 ' .
- R 3 , R 4 , R5, R 6 , R 7 , R % , R 10 and Rn are all H.
- n, n' , and n" are independently an integer from 1 to 5.
- U is represented by the followin formula:
- V in each of the formulas designated in the above alternative embodiments is H or N0 2 .
- LI , LI', D, L2 and L2' do not comprise a self-immolative spacer moiety.
- LI , LI', D, L2 and L2' do not comprise a self-immolative spacer moiety connected to a peptide moiety.
- LI and LI ' do not comprise a self-immolative spacer moiety which spaces and covalently links together the drug moiety and a protein peptide moiety (in the case of LI) or the reactive functional group in LI' and a protein peptide moiety (in the case of LI').
- a self-immolative spacer may be defined as a bifunctional chemical moiety which is capable of covalently linking together two spaced chemical moieties into a normally stable tripartate molecule, releasing one of said spaced chemical moieties from the tripartate molecule by means of enzymatic cleavage; and following said enzymatic cleavage, spontaneously cleaving from the remainder of the molecule to release the other of said spaced chemical moieties.
- Such enzymatic cleavage will activate the self-immolating character of the spacer moiety and initiate spontaneous cleavage of the bond covalently linking the spacer moiety to rest of the molecule, e.g., the drug moiety, to thereby effect release of the drug in pharmacologically active form.
- T is O, N or S
- T is O, N or S
- Rl is C1-C5 alkyl
- T is O, N or S
- R2 is H or Cl-C5 alkyl
- T is O, N or S
- T is O, S or N.
- C1-C5 alkyl is meant to include a branched or unbranched hydrocarbon chain having, unless otherwise noted, one to five carbon atoms, including but not limited to methyl, ethyl, isopropyl, n-propyl, sec-butyl, isobutyl, n-butyl and the like. Additional Examples are shown below
- these groups do not comprise the peptides gly-phe-leu-gly and ala-leu-ala-leu.
- conjugates of the present invention can be prepared according to any known method in the art. See, for example, WO 2009/134977, U.S. Patent Nos. 7,811,572,
- the conjugates of the present invention can be prepared by reacting a cell-binding agent with a cytotoxic compound of the invention ⁇ e.g., cytotoxic compounds of Formula (II)) having a reactive moiety capable of forming a covalent bond with the cell-binding agent to form a cell- binding agent-cytotoxic agent conjugate.
- the conjugate can optionally be purified.
- the cytotoxic compound of the invention can be generated in situ and used to react with the antibody without purification. Alternatively, the cytotoxic compound of the invention can be generated and purified before conjugating to the cell-binding agent.
- the conjugates of the present invention can be prepared by: a) reacting a cell-binding agent with a reactive functional group attached to one of the spacers linked to a branched scaffold to form a modified cell-binding agent ⁇ e.g., that of formula (IV)); b) optionally purifying the modified cell-binding agent; c) conjugating a cytotoxic drug / chemotherapeutic agent to the modified cell-binding agent to form the cell-binding agent- cytotoxic compound conjugate of the present invention; and d) purifying the cell-binding agent-cytotoxic compound conjugate.
- the conjugate of the present invention can be prepared by mixing together a cell-binding agent, a cytotoxic drug / chemotherapeutic agent, and a linker compound (e.g., that of formula (III)).
- the cell-binding agent may be contacted with a cytotoxic drug / chemotherapeutic agent first to form a mixture comprising the cell- binding agent and the cytotoxic drug / chemotherapeutic agent, followed by contacting the mixture with a linker compound (e.g., linker compounds of Formula (III)) to form the cell- binding agent-cytotoxic compound conjugate.
- the conjugate can then be purified.
- the conjugates of the present invention can be purified using tangential flow filtration (TFF), non-adsorptive chromatography, adsorptive chromatography, adsorptive filtration, selective precipitation, high performance liquid chromatography (HPLC), dialysis or any other suitable purification process, as well as combinations thereof.
- THF tangential flow filtration
- HPLC high performance liquid chromatography
- TFF systems Any suitable TFF systems may be utilized for purification, including a Pellicon type system (Millipore, Billerica, MA), a Sartocon Cassette system (Sartorius AG, Edgewood, NY), and a Centrasette type system (Pall Corp., East Hills, NY).
- Pellicon type system Millipore, Billerica, MA
- Sartocon Cassette system Sartorius AG, Edgewood, NY
- Centrasette type system Pall Corp., East Hills, NY.
- Any suitable adsorptive chromatography resin may be utilized for purification.
- Preferred adsorptive chromatography resins include hydroxyapatite chromatography, hydrophobic charge induction chromatography (HCIC), hydrophobic interaction
- hydroxyapatite resins include ceramic hydroxyapatite (CHT Type I and Type II, Bio-Rad Laboratories, Hercules, CA), HA Ultrogel hydroxyapatite (Pall Corp., East Hills, NY), and ceramic fluoroapatite (CFT Type I and Type II, Bio-Rad
- HCIC resin An example of a suitable HCIC resin is MEP Hypercel resin (Pall Corp., East Hills, NY).
- suitable HIC resins include Butyl-Sepharose, Hexyl-Sepaharose, Phenyl-Sepharose, and Octyl Sepharose resins (all from GE Healthcare, Piscataway, NJ), as well as Macro-prep Methyl and Macro-Prep t-Butyl resins (Biorad Laboratories, Hercules, CA).
- Suitable ion exchange resins include SP- Sepharose, CM-Sepharose, and Q-Sepharose resins (all from GE Healthcare, Piscataway, NJ), and Unosphere S resin (Bio-Rad Laboratories, Hercules, CA).
- suitable mixed mode ion exchangers include Bakerbond ABx resin (JT Baker, Phillipsburg NJ).
- IMAC resins examples include Chelating Sepharose resin (GE Healthcare, Piscataway, NJ) and Profinity IMAC resin (Bio-Rad Laboratories, Hercules, CA).
- suitable dye ligand resins include Blue Sepharose resin (GE Healthcare, Piscataway, NJ) and Affi-gel Blue resin (Bio-Rad Laboratories, Hercules, CA).
- suitable affinity resins include Protein A Sepharose resin (e.g., MabSelect, GE Healthcare, Piscataway, NJ), His-Tag metal affinity resins, anti-FLAG affinity resins, and lectin affinity resins, e.g.
- Lentil Lectin Sepharose resin (GE Healthcare, Piscataway, NJ), where the antibody bears appropriate lectin binding sites.
- suitable reversed phase resins include C4, C8, and C18 resins (Grace Vydac, Hesperia, CA).
- any suitable non-adsorptive chromatography resin may be utilized for purification.
- size-exclusion chromatography can be used for purifying the conjugates of the invention.
- suitable non-adsorptive chromatography resins include, but are not limited to, SEPHADEXTM G-25, G-50, G-100, SEPHACRYLTM resins (e.g. , S-200 and S- 300), SUPERDEXTM resins (e.g., SUPERDEXTM 75 and SUPERDEXTM 200), BIO-GEL® resins (e.g., P-6, P-10, P-30, P-60, and P- 100), and others known to those of ordinary skill in the art.
- the conjugate when the cell-binding agent is an epitope-tagged Avibody, the conjugate can be purified using hydroxyl apatite chromatography, size-exclusion
- affinity chromatography specifically affinity chromatography, more specifically His-tag metal affinity chromatography and anti-FLAG M2 affinity chromatography (see, for example, US 2008/0152586 and US 2012/0171115).
- the conjugate when the cell-binding agent is a Centyrin, can be purified using protein A purification, ammonium sulfate or ethanol precipitation, acid extraction, anion or cation exchange chromatography, phosphocellulose chromatography, hydrophobic interaction chromatography, size-exclusion chromatography, tangential flow filtration, affinity chromatography, hydroxylapatite chromatography and lectin
- the conjugate can be purified using HPLC.
- the conjugate can be purified by using affinity chromatography, more specifically His-tag metal affinity chromatography. See, for example, US 2010/0255056, US 2010/0216708 and US 2011/0274623.
- the conjugate when the cell-binding agent is a DARPin, can be purified by affinity chromatography, size exclusion chromatography, hydroxylapatite chromatography, tangential flow filtration, specifically affinity chromatography, more specifically His-Tag affinity chromatography. See, for example, U.S. Patent Publication Nos. 2004/0132028, 2009/0082274, 2011/0118146, and 2011/0224100, WO 02/20565 and WO 06/083275.
- the number of cytotoxic compound molecule bound per cell-binding agent (e.g. , antibody) molecule can be determined spectroscopically by measuring the ratio of the absorbance at 280 nm and 252 nm. An average of about 0.5 - about 20 cytotoxic
- the average number of linked cytotoxic compound per cell-binding agent in the conjugate is about 0.5 to about 10, about 0.5 to 2 (e.g., 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, or 2.1), about 2 to about 8 (e.g., 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 3.0, 3.1, 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9,
- the cytotoxic compounds and cell-binding agent-drug conjugates of the invention can be evaluated for their ability to suppress proliferation of various cancer cell lines in vitro.
- the in vitro cytotoxicity assays can be conducted using methods known in the art (e.g. , Widdison, W. C. et al., "Semisynthetic maytansine analogues for the targeted treatment of cancer," J. Med. Chem. (2006) 49(14):4392-408).
- cells to be evaluated can be exposed to the compounds or conjugates for 1-5 days and the surviving fractions of cells measured in direct assays by known methods. IC 50 values can then be calculated from the results of the assays.
- the present invention includes a composition (e.g. , a pharmaceutical composition) comprising conjugates (e.g., conjugates of Formula (I), or cytotoxic compounds (e.g. , cytotoxic compounds of Formula (II)) described herein, and a carrier (a pharmaceutically acceptable carrier).
- the present invention also includes a composition (e.g., a pharmaceutical composition) comprising the conjugate of Formula (I), or the cytotoxic compound of Formula (II), and a carrier (a pharmaceutically acceptable carrier), and further comprising a second therapeutic agent.
- the present compositions are useful for inhibiting abnormal cell growth or treating a proliferative disorder in a mammal (e.g. , human).
- compositions are also useful for treating an autoimmune disorder, a destructive bone disorder, a graft versus host disease, a transplant rejection, an immune deficiency, an inflammatory disease, an infectious disease, a viral disease, a fibrotic disease, a neurodegenerative disorder, a pancreatitis or kidney disease in a mammal (e.g., human).
- the present invention includes a method of inhibiting abnormal cell growth or treating a proliferative disorder in a mammal (e.g. , human) comprising administering to said mammal a therapeutically effective amount of conjugates (e.g., conjugates of formula (I)) or cytotoxic compounds (e.g., compounds of formula (II)) described herein, or a composition thereof, alone or in combination with a second therapeutic agent.
- a mammal e.g., human
- conjugates e.g., conjugates of formula (I)
- cytotoxic compounds e.g., compounds of formula (II)
- the proliferative disorder is cancer in general; alternatively, the proliferative disorder is cancer selected from the group consisting of breast cancer, colon cancer, brain cancer, prostate cancer, kidney cancer, pancreatic cancer, ovarian cancer, head and neck cancer, melanoma, colorectal cancer, gastric cancer, squamous cancer, small-cell lung cancer, nonsmall-cell lung cancer, testicular cancer, Merkel cell carcinoma, glioblastoma, neuroblastoma, a cancer of a lymphatic organ, and a hematological malignancy.
- the present invention provides a method for inducing cell death in selected cell populations comprising contacting target cells or tissue containing target cells with an effective amount of the conjugates of the present invention.
- the target cells are cells to which the cell-binding agent of the conjugates can bind.
- chemotherapeutic drugs that are therapeutically effective will depend on the particular cancer being treated, the extent of the disease and other factors familiar to the physician of skill in the art and can be determined by the physician.
- the contents of the PDR are expressly incorporated herein in its entirety by reference.
- One of skill in the art can review the PDR, using one or more of the following parameters, to determine dosing regimen and dosages of the chemotherapeutic agents and conjugates that can be used in accordance with the teachings of this invention. These parameters include: Comprehensive index; Manufacturer; Products (by company's or trademarked drug name); Category index; Generic/chemical index (non- trademark common drug names); Color images of medications; Product information, consistent with FDA labeling; Chemical information; Function/action; Indications &
- the present invention also provides methods of treating a non-cancerous condition comprising administering to a subject in need of treatment an effective amount of any of the conjugates described above.
- the conditions include, but not limited to, an autoimmune disorder (e.g., systemic lupus, rheumatoid arthritis, and multiple sclerosis), a graft versus host disease, a transplant rejection (e.g., a renal transplant rejection, a liver transplant rejection, a lung transplant rejection, a cardiac transplant rejection, and a bone marrow transplant rejection), an immune deficiency, an inflammatory diseases (i.e., myositis and pancreatitis), a destructive bone disorder, an infectious disease (e.g., viral infections and parasite infections), a viral disease, a fibrotic disease, a neurodegenerative disorder, or a kidney disease.
- the condition selected from the group consisting of cancer, rheumatoid arthritis, multiple sclerosis, graft versus host disease,
- Examples of in vitro uses include treatments of autologous bone marrow prior to their transplant into the same patient in order to kill diseased or malignant cells: treatments of bone marrow prior to their transplantation in order to kill competent T cells and prevent graft- versus-host-disease (GVHD); treatments of cell cultures in order to kill all cells except for desired variants that do not express the target antigen; or to kill variants that express undesired antigen.
- treatments of autologous bone marrow prior to their transplant into the same patient in order to kill diseased or malignant cells treatments of bone marrow prior to their transplantation in order to kill competent T cells and prevent graft- versus-host-disease (GVHD); treatments of cell cultures in order to kill all cells except for desired variants that do not express the target antigen; or to kill variants that express undesired antigen.
- GVHD graft- versus-host-disease
- Examples of clinical ex vivo use are to remove tumor cells or lymphoid cells from bone marrow prior to autologous transplantation in cancer treatment or in treatment of autoimmune disease, or to remove T cells and other lymphoid cells from autologous or allogenic bone marrow or tissue prior to transplant in order to prevent GVHD.
- Treatment can be carried out as follows. Bone marrow is harvested from the patient or other individual and then incubated in medium containing serum to which is added the cytotoxic agent of the invention, concentrations range from about 10 ⁇ to 1 pM, for about 30 minutes to about 48 hours at about 37°C. The exact conditions of concentration and time of incubation, i.e., the dose, are readily determined by one of ordinary skill in the art.
- the bone marrow cells After incubation the bone marrow cells are washed with medium containing serum and returned to the patient intravenously according to known methods. In circumstances where the patient receives other treatment such as a course of ablative chemotherapy or total-body irradiation between the time of harvest of the marrow and reinfusion of the treated cells, the treated marrow cells are stored frozen in liquid nitrogen using standard medical equipment.
- the cytotoxic compounds or conjugates of the invention will be supplied as a solution or a lyophilized powder that are tested for sterility and for endotoxin levels.
- suitable protocols of conjugate administration are as follows.
- Conjugates are given weekly for 4 weeks as an intravenous bolus each week.
- Bolus doses are given in 50 to 1000 ml of normal saline to which 5 to 10 ml of human serum albumin can be added. Dosages will be 10 ⁇ g to 2000 mg per administration, intravenously (range of 100 ng to 20 mg/kg per day). After four weeks of treatment, the patient can continue to receive treatment on a weekly basis. Specific clinical protocols with regard to route of
- administration can be determined by one of ordinary skill in the art as the clinical situation warrants.
- Suitable pharmaceutically acceptable carriers, diluents, and excipients are well known and can be determined by those of ordinary skill in the art as the clinical situation warrants.
- suitable carriers, diluents and/or excipients include: (1) Dulbecco's phosphate buffered saline, pH about 7.4, containing or not containing about 1 mg/ml to 25 mg/ml human serum albumin, (2) 0.9% saline (0.9% w/v NaCl), and (3) 5% (w/v) dextrose; and may also contain an antioxidant such as tryptamine and a stabilizing agent such as Tween 20.
- the method for inducing cell death in selected cell populations can be practiced in vitro, in vivo, or ex vivo.
- cytotoxic agents [232]
- each of the cytotoxic agents described herein can be modified in such a manner that the resulting compound still retains the specificity and/or activity of the starting compound.
- the skilled artisan will also understand that many of these compounds can be used in place of the cytotoxic agents described herein.
- the cytotoxic agents of the present invention include analogues and derivatives of the compounds described herein.
- FMoc-Lys(FMoc)-OH (2.171 g, 3.68 mmol) and O-Benzotriazole- ⁇ , ⁇ , ⁇ ', ⁇ '-tetramethyl-uronium-hexafluoro-phosphate (HBTU, 3.48 g, 9.19 mmol) were dissolved in dimethylformamide (80 mL). This solution was transferred to the reaction vessel containing the resin, and treated with diisopropyl ethylamine (DIPEA, 3.20 ml, 18.4 mmol). The suspension was agitated for 2h then resin was washed with dimethyl formamide (4X lOOmL) followed by washing with dichloromethane (2X 100 mL).
- DIPEA diisopropyl ethylamine
- Resin was dried then treated with piperidine 20% in dimethylformamide (100 ml, 3.68 mmol) and allowed to agitate for 40 min then drained and washed with dimethylformamide (4X lOOmL) while agitating resin.
- the resin was drained then SPDP (1.15 g, 3.68 mmol) and 1- hydroxybenzotriazol (HOBT, 3.5 g, 9.20 mmol) were dissolved in dimethylformamide (50 mL) and added to the reaction vessel followed by diisopropyl ethylamine (3.2 mL, 18.40 mmol) and agitated at room temperature for 3 hrs. in the dark.
- Resin was washed with dimethylformamide (4X 50mL) followed by washing with dichloromethane (2X 50 mL). The resin was then washed with methanol (100 mL). The reaction vessel was attached to a vacuum flask which was placed under vacuum overnight in the dark. A solution of 95:5 trifluoroacetic acid:deionized water (100 ml) was added to the dry resin with agitation for 4hrs. The reaction was vacuum filtered and filtrate was collected and the filter cask was washed with 95:5 trifluoroacetic acid:deionized water (2x 100 mL) under vacuum. The filtrates were combined and solvent was evaporated under vacuum.
- Residue was taken up in a minimum volume of dimethylformamide then purified by on an intelliflash system using a 45- g CI 8 cartridge flow rate 50 mL/min eluting with deionized water with the following gradient starting at 5% acetonitrile for 5 min and a linear ramp to 95% acetonitrile over the next 25 min. desired product fractions eluting between 18-20 min was frozen then lyophilized to give 289 mg, (12% yield) of desired product. MS [M+1] calcd. 611.8 found.
- reaction was purified by preparative reverse phase HPLC using a kromasil C18 10 micron 250 x 21.2 mm, flow rate 20 mL/min eluting with deionized water containing 0.1% formic acid with a linear gradient of 5% acetonitrile to 95% acetonitrle over 18 min. Fractions containing pure desired product (R t 11.8 min) were immediately combined, frozen and lyophilized give 88.4 mg (26.4 % yield) of desired product. MS [M+1] calcd.709.9 found 709.4.
- Fmoc-Glu-OH (317 mg, 0.859 mmol) was dissolved in a minimal volume of dimethylformamide and treated with l-ethyl-3-(3-dimethylaminopropyl)carbodiimide) HC1 salt (EDC, 823 mg, 4.29 mmol) and l-Hydroxy-7-azabenzotriazole (HOAt, 580 mg, 4.29 mmol) followed by a solution of 2-(pyridin-2-yldisulfanyl)ethanamine (400mg, 2.147 mmol) in a minimal volume of dimethylformamide. After 3h the reaction was purified on an l-ethyl-3-(3-dimethylaminopropyl)carbodiimide) HC1 salt (EDC, 823 mg, 4.29 mmol) and l-Hydroxy-7-azabenzotriazole (HOAt, 580 mg, 4.29 mmol) followed by a solution of
- FMoc-Glu(amido-ethyl-2-sulfanyl DM4)-amidoethyl-s-sulfanyl-DM4 (89.6mg, 0.044 mmol) was treated with 20% morpholine in dimethylformamide (2 mL) and allowed to stir under argon at room temperature. After 2 h the material was purified by HPLC using a 250 x 21.2 mm C8, 10 micron, Kromasil column eluting at a flow rate of 20 mL/min with deionized water containing 0.1% formic acid with a gradient of between 0%-95% acetonitrile over 18 min.
- H-Glu(amido-ethyl-2-sulfanyl DM4)-amidoethyl-s-sulfanyl-DM4) (65mg, 0.036 mmol) was dissolved in a minimal volume of dimethylformamide and treated with BMPS (13.39 mg, 0.054 mmol) followed by diisopropyl ethylamine (9.35 ⁇ , 0.054 mmol). After 2 h the material was purified by HPLC using a 250 x 21.2 10 micron, Kromasil column, eluting at 20 mL/min with deionized water using a gradient of between 5% - 95% acetonitrile over 18 min.
- H-Glu(OtBu)-OtBu HCl salt (556 mg, 1.878 mmol) was dissolved in anhydrous dimethylformamide and treated with diisopropyl ethylamine (656 ⁇ , 3.76 mmol) followed by a solution of BMPS (500 mg, 1.878 mmol) in dimethylformamide (6 mL) and allowed to proceed at room temp under argon.
- Fmoc-Gly 3 -OH (285 mg, 0.692 mmol) was dissolved in a minimal volume of dimethylformamide and treated with l-ethyl-3-(3-dimethylaminopropyl)carbodiimide) HCl salt (EDC, 442 mg, 2.307 mmol) and a solution of MayNMA (300mg, 0.461 mmol) in dimethylformamide. MayNMA was in turn prepared as described in US 7,598,375. The reaction was allowed to proceed at room temperature under argon for 6 h.
- the cells were resuspended by gentle vortex in a plate with a cover and washed with 200 ⁇ of cold FACS Buffer. The washing solution was removed by repeating the centrifuge step.
- FITC labeled secondary antibody was diluted to 1: 100 in FACS Buffer and 100 ⁇ ⁇ of the diluted antibody was added to each well on the assay plate. The plate was incubated for 1 hour on ice without exposing to light and then spun down and washed as described above. The cells were resuspended in 200 ⁇ ⁇ of Fixer (lxPBS with 1% Formaldehyde). The reading was taken on the FACS Calibur. As shown in Figures 24 and 25, the conjugates bind equally well as the naked antibody with similar Kd values.
- the conjugate containing a branched linking group (Ab-linker2-(DM4) 2 or Ab-linker3-(MayNMA) 2 ) is more potent than a conjugate with non-branched linking group, Ab-SPDB-DM4.
- the potency of the conjugates significantly decreases when the cells were blocked with naked antibodies, indicating target- specific cytotoxicity of the conjugates.
- N-hydroxysuccinimide ester of Z-Lys(Z)-OH Z-Lys(Z)-OH (2g, 4.83mmol) was dissolved in dimethylformamide (10 mL) and treated with l-hydroxypyrrolidine-2,5-dione (0.833 g, 7.24 mmol) followed by EDC (0.925 g, 4.83 mmol). The reaction was allowed to proceed at room temperature, under argon atmosphere overnight to give crude N- hydroxysuccinimide ester which was used without purification.
- Z-Lys(Z)-Cysteic Acid Cysteic Acid (2.434 g, 14.47 mmol) was weighed into a 250 mL beaker equipped with a stir bar and dissolved in a solution of deionized water (70 ml) and dimethylformamide (30 ml). The mixture was stirred while 1M NaOH was added to bring the pH to 8.4. Crude Z-Lys(Z)-NHS (2.468 g, 4.82 mmol) in dimethylformamide (1 mL) was added dropwise while keeping the pH between 7.5-8.4 with the addition of 1M NaOH. After addition the reaction was allowed to stir for lh.
- Lys-Cys-P-Ala-OH Z-Lys(Z)-Cysteic Acid- -Ala-OtBu (497mg, 0.718 mmol) was treated with 95:5 TFA:Water (10 mL) under an argon atmosphere overnight. The material was co- evaporated with toluene (3x 5 mL). The crude product was purified purified using a
- SPDB-Lys(SPDB)-Cys-P-Ala Lys-Cys- -Ala-OH (37mg, 0.101 mmol) was dissolved in dimethyl sulfoxide ( 1 mL) and treated with a solution of SPDB (72.3 mg, 0.222 mmol) dissolved in dimethyl sulfoxide (1 mL) and followed by diisopropyl ethylamine (0.035 ml, 0.201 mmol). The reaction was allowed to proceed at room temperature under an Argon atmosphere for 3h then was purified by semi-preparative C18 HPLC using a XB-C18 21.2 x 150mm, 5 ⁇ column with a flow rate of 21.2mL/min.
- Z-Lys(Z)-b-Ala-OtBu Z-L-Lys(Z)-OH (10.0 g, 24 mmol) was dissolved in dimethyl formamide (100 mL) to which was added beta-alanine t-butyl ester HCl salt (5.0 g, 27 mmol), EDC (6.0 g, 31 mmol) and HOBT (4.0 g, 25 mmol). After reacting for 2 h the reaction was purified directly on a 1200 g C18 cartridge on an intelliflash system, eluting with deionized water containing 0.2% formic acid with a gradient of 5 - 95% acetonitrile over 50 min at a flow rate of 100 mL/min.
Abstract
The invention provides a branched linker compound with reactive moieties for forming covalent bonds with multiple cytotoxic agents (drugs) and/or a cell-binding agent (CBA); a cytotoxic compound comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates; a modified cell-binding agent comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates; and conjugates so formed. Such conjugates and/or cytotoxic compounds may be effective for treating a range of diseases, such as cancer, with a relatively high activity at a relatively low, non-toxic dose.
Description
CONJUGATES COMPRISING CELL-BINDING AGENTS AND CYTOTOXIC
AGENTS
REFERENCE TO RELATED APPLICATIONS
[01] This application claims priority to and the benefit of the filing date of U.S. provisional application No. 61/829,893, filed on May 31, 2013, the entire content of which is
incorporated herein by reference.
BACKGROUND OF THE INVENTION
[02] Antibody-drug conjugates (ADC) and cell binding agent-drug conjugates are emerging as a powerful class of anti-tumor agents with efficacy across a range of cancers. The cell binding agent-drug conjugates (such as ADCs) are commonly composed of three distinct elements: a cell-binding agent (e.g., antibody); a linking component; and a cytotoxic moiety. The linking component of ADC is an important element in developing targeted anticancer agents that possess an optimal therapeutic window, i.e., therapeutic activity at a low, non-toxic dose.
[03] Therefore, there is a need for targeted therapies such as ADCs and other cell binding agent-drug conjugates having a new class of linking components.
SUMMARY OF THE INVENTION
[04] A first embodiment of the invention features a conjugate represented by the following formula, or a pharmaceutically acceptable salt thereof:
(I).
[05] In Formula (I) above, CBA is a cell binding agent; hi is a spacer; U is a branched scaffold; L2, for each occurrence, is independently a spacer; D, for each occurrence, is independently a cytotoxic drug moiety; q is an integer from 2 to 5; and w is an integer between 1 and 10.
[06] A second embodiment of the invention features a cytotoxic compound represented by the following formula, or a salt (e.g., a pharmaceutically acceptable salt) thereof:
[07] In Formula (II) above, L is a spacer attached to a reactive functional group that can form a covalent bond with a cell-binding agent; U is a branched scaffold; L2, for each
occurrence, is independently a spacer; D, for each occurrence, is independently a cytotoxic drug moiety; and q is an integer from 2 to 5.
[08] A third embodiment of the invention features a linker compound represented by the following formula, or a salt (e.g., a table salt) thereof:
[09] In Formula (III) above, is a spacer attached to a reactive functional group that can form a covalent bond with a cell-binding agent; U is a branched scaffold; L2', for each occurrence, is independently a spacer attached to a reactive functional group that can form a covalent bond with a cytotoxic agent; and q is an integer from 2 to 5.
[10] A fourth embodiment of the invention features a modified cell binding agent represented by the following formula, or a salt (e.g. , a pharmaceutically acceptable salt) thereof:
[11] In Formula (IV), CBA is a cell binding agent; h is a spacer; U is a branched scaffold;. L2', for each occurrence, is independently a spacer attached to a reactive functional group that can form a covalent bond with a cytotoxic agent; q is an integer from 2 to 5; and w is an integer between 1 and 10.
[12] Also within the scope of this invention is a composition (e.g. , a pharmaceutical composition) comprising a conjugate represented by Formula (I), a cytotoxic compound represented by Formula (II), or a salt (e.g. , a pharmaceutical acceptable salt) thereof. The composition may also include a carrier (e.g. , a pharmaceutically acceptable carrier). The composition can further include a second therapeutic agent.
[13] The present invention also includes a method of inhibiting abnormal cell growth or treating a proliferative disorder, a destructive bone disorder, an autoimmune disorder, a graft versus host disease, a transplant rejection, an immune deficiency, an inflammatory diseases, an infectious disease, a viral disease, a fibrotic disease, a neurodegenerative disorder, pancreatitis, or a kidney disease in a mammal (e.g. , human), comprising administering to said mammal a therapeutically effective amount of a conjugate represented by Formula (I), a cytotoxic compound represented by Formula (II), or a salt (e.g. , a pharmaceutical acceptable salt) thereof.
[14] In a related embodiment, the method described above further comprises administering to said mammal simultaneously, sequentially, or consecutively a second therapeutic (e.g., chemotherapeutic) agent.
BRIEF DESCRIPTION OF THE DRAWINGS
[15] Figure 1 depicts the structures of certain maytansinoids.
[16] Figures 2-6 depict the synthetic schemes for preparing cytotoxic compounds each comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates.
[17] Figures 7 and 8 depict the synthetic schemes for preparing branched linker compounds each with reactive moieties for forming covalent bonds with multiple cytotoxic agents (drugs).
[18] Figures 9 and 10 depict the synthetic schemes for preparing cytotoxic compounds each comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates.
[19] Figure 11 depicts the structure of a cytotoxic compound comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates.
[20] Figure 12 depicts the synthetic scheme for preparing a branched linker compound with reactive moieties for forming covalent bonds with multiple cytotoxic agents (drugs).
[21] Figures 13-16 depict the synthetic schemes for preparing cytotoxic compounds each comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugates.
[22] Figures 17-23 depict the synthetic schemes for preparing branched linker compounds containing branched scaffolds.
[23] Figures 24-25 show cell-binding properties of conjugates having branched linkers (i.e., Ab-linker2-(DM4)2 or Ab-linker3-(MayNMA)2 in comparison with naked antibody.
[24] Figures 26-28 show the cytotoxicity effects of conjugates having branched linkers (Ab-linker2-(DM4)2 and Ab-linker3-(MayNMA)2 in comparison with those having a non- branched linker (Ab-SPDB-DM4) on COLO205 cells, HCT- 15 cells, and RPMI8226. The CBA used in the conjugates is chB38.1. In the plots SPDB-DM4 4.03D/A, Ab-linker2- (DM4)2 3.1D/A, and Ab-linker3-(MayNMA)2 4.26D/A, the cytotoxicity assay was performed in the absence of the competing non-conjugated antibody. In the plots SPDB- DM4 4.03D/A with blocking, Ab-linker2-(DM4)2 3.1D/A with blocking, and Ab-linker3-
(MayNMA)2 4.26D/A with blocking, the cytotoxicity assay was performed in the presence of the competing non-conjugated antibody.
[25] Figure 29 depicts the synthetic scheme for preparing a cytotoxic compound comprising a branched linker moiety with a reactive group for forming a CBA-drug conjugate.
[26] Figure 30 depicts the synthetic scheme for preparing a branched linker compound containing branched scaffolds.
DETAILED DESCRIPTION OF THE INVENTION
[27] Reference will now be made in detail to certain embodiments of the invention, examples of which are illustrated in the accompanying structures and formulas. While the invention will be described in conjunction with the enumerated embodiments, it will be understood that they are not intended to limit the invention to those embodiments. On the contrary, the invention is intended to cover all alternatives, modifications, and equivalents which may be included within the scope of the present invention as defined by the claims. One skilled in the art will recognize many methods and materials similar or equivalent to those described herein, which could be used in the practice of the present invention.
[28] It should be understood that any of the embodiments described herein, including those described under different aspects of the invention (e.g., compounds, conjugates,
compositions, methods of making and using) and different parts of the specification
(including embodiments described only in the Examples) can be combined with one or more other embodiments of the invention, unless explicitly disclaimed or improper. Combination of embodiments are not limited to those specific combinations claimed via the multiple dependent claims.
DEFINITIONS
[29] "Alkyl" as used herein refers to a saturated linear or branched-chain monovalent hydrocarbon radical of one to twenty carbon atoms. "Monovalent" means that alkyl has one point of attachment to the remainder of the molecule. Examples of alkyl groups include, but are not limited to, methyl, ethyl, 1 -propyl, 2-propyl, 1 -butyl, 2-methyl-l -propyl, - CH2CH(CH3)2, 2-butyl, 2-methyl-2-propyl, 1-pentyl, 2-pentyl, 3-pentyl, 2-methyl-2-butyl, 3- methyl-2-butyl, 3-methyl- l -butyl, 2-methyl-l -butyl, 1-hexyl, 2-hexyl, 3-hexyl, 2-methyl-2- pentyl, 3-methyl-2-pentyl, 4-methyl-2-pentyl, 3-methyl-3-pentyl, 2-methyl-3-pentyl, 2,3- dimethyl-2-butyl, 3,3-dimethyl-2-butyl, 1-heptyl, 1-octyl, and the like. Specifically, the alkyl group has one to ten carbon atoms. More specifically, the alkyl group has one to four carbon
atoms.
[30] "Alkylene" as used herein refers to a saturated linear or branched-chain divalent hydrocarbon radical of one to twenty carbon atoms, examples of which include, but are not limited to, those having the same core structures of the alkyl groups as exemplified above. "Divalent" means that the alkylene has two points of attachment to the remainder of the molecule. Specifically, the alkylene group has one to ten carbon atoms. More specifically, the alkylene group has one to four carbon atoms.
[31] The terms "carbocycle," "carbocyclyl," carbocyclic and "carbocyclic ring" refer to a monovalent non-aromatic, saturated or partially unsaturated ring having 3 to 12 carbon atoms as a monocyclic ring or 7 to 12 carbon atoms as a bicyclic ring. Bicyclic carbocycles having 7 to 12 atoms can be arranged, for example, as a bicyclo [4,5], [5,5], [5,6] or [6,6] system, and bicyclic carbocycles having 9 or 10 ring atoms can be arranged as a bicyclo [5,6] or [6,6] system, or as bridged systems such as bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane and bicyclo[3.2.2]nonane. Examples of monocyclic carbocycles include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, 1-cyclopent- I-enyl, l-cyclopent-2-enyl, 1-cyclopent- 3-enyl, cyclohexyl, 1-cyclohex-I-enyl, l-cyclohex-2-enyl, l-cyclohex-3-enyl,
cyclohexadienyl, cycloheptyl, cyclooctyl, cyclononyl, cyclodecyl, cycloundecyl,
cyclododecyl, and the like.
[32] The term "cycloalkylalkyl" refers to a cycloalkyl group that is connected to another group by an alkylene group. Examples of cycloalkylalkyls include, but are not limited to, cyclohexylmethyl, cyclohexylethyl, cyclopentylmethyl, cyclopentylethyl, and the like.
Specifically, cycloalkylalkyl is cyclohexylmethyl.
[33] As used herein, an integer "between" x and y includes integers x and y unless otherwise specified to the contrary. For example, "an integer between 1 and 5" can be 1, 2, 3, 4, or 5.
[34] The terms "cyclic alkyl" and "cycloalkyl" can be used interchangeably. They refer to a monovalent saturated carbocyclic ring radical. "Monovalent" means that cycloalkyl has one point of attachment to the remainder of the molecule. The saturated carbocyclic ring can be monocyclic or bicyclic (fused, bridged, or spiro bicyclic). Specifically, the cycloalkyl is 3 to 7 membered monocyclic ring radical. Bicyclic cycloalkyl having 7 to 12 atoms can be arranged, for example, as a bicyclo [4,5], [5,5], [5,6], or [6,6] system, and bicyclic cycloalkyl having 9 or 10 ring atoms can be arranged as a bicyclo [5,6] or [6,6] system, or as bridged systems such as bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane and bicyclo[3.2.2]nonane.
Examples of monocyclic cycloalkyl groups include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl, cyclodecyl, cycloundecyl, cyclododecyl, and the like. More specifically, the cycloalkyl is cyclohexyl.
[35] "Cycloalkylene" as used herein refers to a divalent saturated carbocyclic ring radical having 3 to 12 carbon atoms as a monocyclic ring, or 7 to 12 carbon atoms as a bicyclic ring. "Divalent" means that the cycloalkylene has two points of attachment to the remainder of the molecule. Specifically, the cycloalkylene is a 3- to 7-membered monocyclic. Examples of cyclic alkylene groups include, but not limited to, those having the same core structures of the cylcolakyl groups as exemplified above. More specifically, the cycloalkylene group is cyclohexylene.
[36] "Alkenyl" as used herein refers to linear or branched-chain monovalent hydrocarbon radical of two to twenty carbon atoms with at least one carbon-carbon double bond, wherein the alkenyl radical includes radicals having "cis" and "trans" orientations, or by an alternative nomenclature, "E" and "Z" orientations. "Monovalent" means that alkenyl has one point of attachment to the remainder of the molecule. Examples include, but are not limited to, ethylenyl or vinyl (-CH=CH2), allyl (-CH2CH=CH2), and the like. Specifically, the alkenyl has two to ten carbon atoms, also referred to as "C2-1o alkenyl." More
specifically, the alkenyl has two to four carbon atoms, also referred to as "C2_4 alkenyl."
[37] "Alkenylene" as used herein refers to linear or branched-chain divalent hydrocarbon radical of two to twenty carbon atoms with at least one carbon-carbon double bond, wherein the alkenyl radical includes radicals having "cis" and "trans" orientations, or by an alternative nomenclature, "E" and "Z" orientations. "Divalent" means that the alkenylene has two points of attachment to the remainder of the molecule. Examples include, but are not limited to, ethylenylene or vinylene (-CH=CH-), allylene (-CH2CH=CH-), and the like.
Specifically, the alkenylene has two to ten carbon atoms, also referred to as "C2-1o
alkenylene." More specifically, the alkenylene has two to four carbon atoms, also referred to as "C2_4 alkenylene."
[38] The terms "cyclic alkenyl" and "cycloalkenyl" can be used interchangeably. They refer to a monovalent carbocyclic ring radical with at least one carbon-carbon double bond, having 3 to 12 carbon atoms as a monocyclic ring, or 7 to 12 carbon atoms as a bicyclic ring (fused, bridged, or spiro bicyclic). "Monovalent" means that cycloalkenyl has one point of attachment to the remainder of the molecule. Specifically, the cycloalkenyl is 3 to 7 membered monocyclic ring radical. Bicyclic cycloalkenyl having 7 to 12 atoms can be arranged, for example, as a bicyclo [4,5], [5,5], [5,6], or [6,6] system, and bicyclic cycloalkenyl having 9 or 10 ring atoms can be arranged as a bicyclo [5,6] or [6,6] system, or
as bridged systems such as bicyclo[2.2.1]heptene, bicyclo[2.2.2]octene and
bicyclo[3.2.2]nonene. Examples of the monocyclic alkenyl include, but are not limited to, cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, cyclooctenyl, cyclononenyl, cyclodecenyl, cycloundecenyl, cyclododecenyl, and the like. More
specifically, the cycloalkenyl is cyclohexenyl.
[39] "Cycloalkenylene" as used herein refers to a divalent unsaturated carbocyclic ring radical having 3 to 12 carbon atoms as a monocyclic ring, or 7 to 12 carbon atoms as a bicyclic ring. "Divalent" means that the cycloalkenylene has two points of attachment to the remainder of the molecule. Specifically, the cycloalkenylene is a 3- to 7-membered monocyclic. Examples of cyclic alkenylene include, but not limited to, those having the same core structures of the cylcolakyl groups as exemplified above. More specifically, the cycloalkenylene group is cyclohexenylene.
[40] "Alkynyl" as used herein refers to linear or branched-chain monovalent hydrocarbon radical of two to twenty carbon atoms with at least one carbon-carbon triple bond.
"Monovalent" means that alkynyl has one point of attachment to the remainder of the molecule. Examples include, but are not limited to ethynyl, propynyl, 1-butynyl, 2-butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, hexynyl, and the like. Specifically, the alkynyl has two to ten carbon atoms, also referred to as "C2-1o alkynyl." More specifically, the alkynyl has two to four carbon atoms, also referred to as "C2-4 alkynyl."
[41] "Alkynylene" as used herein refers to an linear or branched-chain divalent hydrocarbon radical of two to twenty carbon atoms with at least one carbon-carbon triple bond, examples of which include, but are not limited to, those having the same core structures of the alkynyl groups as exemplified above. "Divalent" means that the alkynylene has two points of attachment to the remainder of the molecule. Specifically, the alkynylene group has one to ten carbon atoms. More specifically, the alkynylene group has one to four carbon atoms.
[42] The terms "cyclic alkynyl" and "cycloalkynyl" can be used interchangeably. They refer to a monovalent carbocyclic ring radical with at least one carbon-carbon triple bond, having 8 to 12 carbon atoms as a monocyclic ring, or 11 to 17 carbon atoms as a bicyclic ring (fused, bridged, or spiro bicyclic). "Monovalent" means that cycloalkynyl has one point of attachment to the remainder of the molecule. Specifically, the cycloalkynyl is 8-membered monocyclic ring radical.
[43] "Cycloalkyne" as used herein refers carbocyclic ring having one or more triple bonds. It can be monocyclic, bicyclic or tricyclic; bicyclic and tricyclic can be bridged or
fused. The carbocyclic ring optionally contains one or more double bonds and/or is optionally fused with one or more aromatic (e.g., phenyl ring) or heteroaromatic rings.
Examples of cycloalkyne include, but are not limited to, those described in J. Am. Chem. Soc. (2012) 134:9199-9208; WO 2011/136645, US 2009/0068738, Lang K. et al, J. Am. Chem. Soc. (2012) 134: 10317-10320, for example, cyclooctyne, monofluorocyclooctyne, difluorooctyne, DIFO, DIF02, DIFO3, bicylo[6.1.0]non-4-yne, benzocyclooctyne, difluorobenzocyclooctyne, dibenzocyclooctyne, DIBO, and those described in Debets, M. F. et al., Acc. Chem. Res., (2011) 44(9):805-815; and Gold B. et al., J. Am. Chem. Soc. (2013) 135(4): 1558-1569. Specifically, cycloalkyne is cyclooctyne.
[44] "Cycloalkynylene" as used herein refers to a divalent carbocyclic ring radical having at least one carbon-carbon triple bond. "Divalent" means that the cycloalkynylene has two points of attachment to the remainder of the molecule. Specifically, the cycloalkynylene is a 8 to 14-membered monocyclic.
[45] "Heterocycloalkyne" as used herein refers to a heterocyclic ring having one or more triple bonds. Examples of heterocycloalkyne include, but are not limited to,
dibenzoazacyclooctyne (DIBAC), biarylazacyclooctynone (BARAC), thiacyclooctyne, thiabenzocyclooctyne, thiacycloheptyne and tetramethylthiacycloheptyne.
[46] "Strained cycloalkene" as used herein refers to carbocyclic ring having one trans double bond and 7 to 14 ring atoms. Examples of strained cycloalkene include, but are not limited to, cyclooctene, norbornene and other cycloalkenes described in Debets, M. F. et al., Acc. Chem. Res. (2011) 44(9):805-815.
[47] "Strained heterocycloalkene" as used herein refers to a heterocyclic ring having one trans double bonds and 7 to 14 ring atoms selected from carbon and at least one (typically 1 to 4, more typically 1 or 2) heteroatom (e.g., oxygen, nitrogen or sulfur).
[48] The term "aryl group" means an aromatic hydrocarbon ring system having six to fourteen carbon ring atoms. The term "aryl" may be used interchangeably with the terms "aryl ring" "aromatic ring," "aryl group" and "aromatic group." "Aryl group" also includes an aromatic hydrocarbon ring system fused to a non-aromatic carbocyclic ring system, such as a cycloalkyl group. Examples includes phenyl, naphthyl, anthracenyl, 1,2- dihydronaphthyl, 1,2,3,4-tetrahydronaphthyl, fluorenyl, indanyl, indenyl and the like. An aryl group is monovalent, i.e., has one point of attachment to the remainder of the molecule. A "substituted aryl group" is substituted at any one or more substitutable ring atom, which is a ring carbon atom bonded to a hydrogen."
[49] "Arylene" as used herein refers to a divalent aryl group, i.e., an aryl group having two points of attachment to the remainder of the molecule. "Divalent" means that the arylene has two points of attachment to the remainder of the molecule. Both aryl and arylene groups are sometime represented herein by "Ar." Arylene is specifically phenylene.
[50] "Heteroaryl" (used interchangeably with "heteroaromatic," "heteroaryl ring," "heteroaryl group," "heteroaromatic ring," and "heteroaromatic group") refers to aromatic ring systems having five to fourteen ring atoms selected from carbon and at least one
(typically 1 to 4, more typically 1 or 2) heteroatoms (e.g. , oxygen, nitrogen or sulfur).
"Heteroaryl" includes monocyclic rings and polycyclic rings (e.g. , bicyclic) in which a monocyclic heteroaromatic ring is fused to one or more other aromatic or heteroaromatic rings. As such, "5-14 membered heteroaryl" includes monocyclic, bicyclic or tricyclic ring systems. Heteroaryls are monovalent, meaning that there is one point of attachment to the remainder of the molecule.
[51] "Monocyclic 5-6 membered heteroaryl" means a monocyclic aromatic ring system having five or six ring atoms selected from carbon and at least one (typically 1 to 3, more typically 1 or 2) heteroatoms (e.g. , oxygen, nitrogen or sulfur). Examples of monocyclic 5-6 membered heteroaryl groups include furanyl (e.g., 2-furanyl, 3-furanyl), imidazolyl (e.g., N- imidazolyl, 2-imidazolyl, 4-imidazolyl, 5-imidazolyl), isoxazolyl (e.g., 3-isoxazolyl, 4- isoxazolyl, 5-isoxazolyl), oxadiazolyl (e.g., 2-oxadiazolyl, 5-oxadiazolyl), oxazolyl (e.g., 2- oxazolyl, 4-oxazolyl, 5-oxazolyl), pyrazolyl (e.g. , 3-pyrazolyl, 4-pyrazolyl), pyrrolyl (e.g. , 1- pyrrolyl, 2-pyrrolyl, 3-pyrrolyl), pyridyl (e.g., 2-pyridyl, 3-pyridyl, 4-pyridyl), pyrimidinyl (e.g., 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl), pyridazinyl (e.g., 3-pyridazinyl), thiazolyl (e.g., 2-thiazolyl, 4-thiazolyl, 5-thiazolyl), isothiazolyl, triazolyl (e.g., 2-triazolyl, 5-triazolyl), tetrazolyl (e.g., tetrazolyl), and thienyl (e.g. , 2-thienyl, 3-thienyl). Examples of polycyclic aromatic heteroaryl groups include carbazolyl, benzimidazolyl, benzothienyl, benzofuranyl, isobenzofuranyl, indolyl, benzotriazolyl, benzothiazolyl, benzoxazolyl, quinolinyl, isoquinolinyl, indazolyl, isoindolyl, acridinyl, or benzisoxazolyl. A "substituted heteroaryl group" is substituted at any one or more substitutable ring atom, which is a ring carbon or ring nitrogen atom bonded to a hydrogen.
[52] "Heteroarylene" as used herein refers to a divalent heteroaryl, i.e., a heteroaryl with two points of attachment to the remainder of the molecule.
[53] The terms "heterocycle," "heterocyclyl," "heterocyclic" and "heterocyclic ring" are used interchangeably herein and refer to a saturated or unsaturated non-aromatic 3-12 membered ring radical optionally containing one or more double bonds. It can be
monocyclic, bicyclic, or tricyclic; bicyclic and tricyclic can be bridged or fused. The heterocycle contains 1 to 4 heteroatoms, which may be the same or different, selected from N, O or S. The heterocycle optionally contains one or more double bonds and/or is optionally fused with one or more aromatic (e.g. , phenyl ring) or heteroaromatic rings. "3-7 membered monocyclic heterocycle" means a radical having from 3-7 atoms (including 1-3 heteroatoms) arranged in a monocyclic ring. The term "heterocycle" is intended to include all the possible isomeric forms. A heterocycle may be a monocycle having 3 to 7 ring members (e.g., 2 to 6 carbon atoms and 1 to 4 heteroatoms selected from N, O, P, and S) or a bicycle having 7 to 10 ring members (e.g., 4 to 9 carbon atoms and 1 to 6 heteroatoms selected from N, O, P, and S), for example: a bicyclo [4,5], [5,5], [5,6], or [6,6] system. Heterocycles are described in Paquette, Leo A., Principles of Modern Heterocyclic Chemistry (W. A. Benjamin, New York, 1968), particularly Chapters 1, 3, 4, 6, 7, and 9; The Chemistry of Heterocyclic Compounds, A Series of Monographs (John Wiley & Sons, New York, 1950 to present), in particular Volumes 13, 14, 16, 19, and 28; and /. Am. Chem. Soc. (1960) 82:5566.
[54] Examples of heterocyclic rings include, but are not limited to, aziridinyl, pyrrolidinyl, tetrahydrofuranyl, dihydrofuranyl, tetrahydrothienyl, tetrahydropyranyl, dihydropyranyl, tetrahydrothiopyranyl, tetrahydropyrrolyl, piperidino, morpholino, thiomorpholino, thioxanyl, piperazinyl, homopiperazinyl, azetidinyl, oxetanyl, thietanyl, homopiperidinyl, oxepanyl, thiepanyl, oxazepinyl, diazepinyl, thiazepinyl, 2-pyrrolinyl, 3-pyrrolinyl, indolinyl, isoindolinyl, 2H-pyranyl, 4H-pyranyl, dioxanyl, 1,3-dioxolanyl, pyrazolinyl, dithianyl, dithiolanyl, dihydropyranyl, dihydrothienyl, dihydrofuranyl, pyrazolidinyl, imidazolinyl, imidazolidinyl, 3-azabicyco[3.1.0]hexanyl, 3-azabicyclo[4.1.0]heptanyl, and
azabicyclo[2.2.2]hexanyl. Spiro moieties are also included within the scope of this definition. Examples of a heterocyclic group wherein ring atoms are substituted with oxo (=0) moieties are pyrimidinonyl and 1, 1-dioxo-thiomorpholinyl.
[55] The heterocycle, heteroaryl, or heteroarylene groups may be carbon (carbon-linked) or nitrogen (nitrogen-linked) attached where such is possible. By way of example and not limitation, carbon bonded heterocycle, heteroaryl or heroarylene groups are bonded at position 2, 3, 4, 5, or 6 of a pyridine, position 3, 4, 5, or 6 of a pyridazine, position 2, 4, 5, or 6 of a pyrimidine, position 2, 3, 5, or 6 of a pyrazine, position 2, 3, 4, or 5 of a furan, tetrahydrofuran, thiophene, pyrrole or tetrahydropyrrole, position 2, 4, or 5 of an oxazole, imidazole or thiazole, position 3, 4, or 5 of an isoxazole, pyrazole, or isothiazole, position 2 or 3 of an aziridine, position 2, 3, or 4 of an azetidine, position 2, 3, 4, 5, 6, 7, or 8 of a quinoline or position 1, 3, 4, 5, 6, 7, or 8 of an isoquinoline.
[56] By way of example and not limitation, nitrogen bonded heterocycle, heteroaryl, or heteroarylene groups are bonded at position 1 of an aziridine, azetidine, pyrrole, pyrrolidine, 2-pyrroline, 3-pyrroline, imidazole, imidazolidine, 2-imidazoline, 3-imidazoline, pyrazole, pyrazoline, 2-pyrazoline, 3-pyrazoline, piperidine, piperazine, indole, indoline, IH-indazole, position 2 of a isoindole or isoindoline, position 4 of a morpholine, and position 9 of a carbazole, or O-carboline.
[57] The heteroatoms present in heteroaryl, heteroarylene, or heterocyclcyl can include the oxidized forms such as NO, SO, and S02.
[58] "Halogen" refers to F, CI, Br or I.
[59] If a group is described as being "optionally substituted," the group may be either (1) not substituted, or (2) substituted. If a carbon of a group is described as being optionally substituted with one or more of a list of substituents, one or more of the hydrogen atoms on the carbon (to the extent there are any) may separately and/or together be replaced with an independently selected optional substituent.
[60] Suitable substituents for an alkyl, alkenyl, alkynyl, cycloalkyl, aryl, heteroaryl, heterocycle, alkylene, alkenylene, alkynylene, cycloalkene, heterocycloalkene, cycloalkyne, heterocycloalkyne, cycloalkylene arylene, and heterarylene are those which do not significantly adversely affect the biological activity of the conjugate. Unless otherwise specified, exemplary substituents for these groups include linear, branched or cyclic alkyl, alkenyl or alkynyl having from 1 to 10 carbon atoms, aryl, heteroaryl, heterocyclyl, halogen, guanidinium [-NH(C=NH)NH2] , -OR100, NR10iRi02, -N02, -NR10iCOR102, -SR100, a sulfoxide represented by -SORioi, a sulfone represented by -S02Rioi, a sulfonate -SO3M, a sulfate - OSO3M, a sulfonamide represented by -S02NRioiRio2, cyano, an azido, -COR10i, -OCORioi, -OCONR1oiRio2 and a polyethylene glycol unit (-OCH2CH2)nR1oi wherein M is H or a cation (such as Na+ or K+); R101, Rio2 and R103 are each independently selected from H, linear, branched or cyclic alkyl, alkenyl or alkynyl having from 1 to 10 carbon atoms, a polyethylene glycol unit (-OCH2CH2)n-R1o4, wherein n is an integer from 1 to 24, an aryl having from 6 to 10 carbon atoms, a heterocyclic ring having from 3 to 10 carbon atoms and a heteroaryl having 5 to 10 carbon atoms; and R104 is H or a linear or branched alkyl having 1 to 4 carbon atoms, wherein the alkyl, alkenyl, alkynyl, aryl, heteroaryl and heterocyclcyl in the groups represented by R10o, R101, Rio2, Rio3 and Rio4 are optionally substituted with one or more (e.g., 2, 3, 4, 5, 6 or more) substituents independently selected from halogen, -OH, -CN, - N02, and unsubstituted linear or branched alkyl having 1 to 4 carbon atoms. Specifically, the substituent for the optionally substituted alkyl, alkylene, cycloalkylene, arylene, and
heteroarylene described above is selected from the group consisting of halogen, -CN, - NR10iRio2, -CF3, -OR10o, aryl, heteroaryl, heterocyclyl, -SR10i, -SOR10i, -S02Rioi, and -S03M. Alternatively, the suitable substituent is selected from the group consisting of - halogen, -OH, -N02, -CN, CM alkyl, -ORioo, NR101R102, -NR101COR102, -SR100, -SO2R101, - SO2NR10iRi02, -COR101, -OCOR10i, and -OCONR10iRi02, wherein R100, R101, and R102 are each independently -H or C1-4 alkyl.
[61] The term "ionizable group" refers to a functional group that can be converted to a charged group by protonation with an acid or deprotonation with a base. Examples of the ionizable groups include -S03H, -Z'-S03H, -OP03H2, -Z'-OP03H2, -P03H2, -Z'-P03H2, - C02H, -Z'C02H, -NRuRi2, or -Z'-NRuRi2, Rn and R12, for each occurrence, are independently H or an optionally substituted alkyl; and Z' includes an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene. In certain embodiments, Z' is alkylene.
[62] The term "charged substituent" refers to a substituent that is either positively or negatively charged. The charge in such a substituent is not removable by treatment with a base or an acid and thus permanent. Examples of the charged substituents include, but not limited to, -N+R13R14R15 and -Z'-N+R13R14R15, in which R1 to R15 are each independently an optionally substituted alkyl; and Z' includes an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene. In certain embodiments, Z' is alkylene.
[63] Charged substituents may contain a counter ion. For positive charged substituents, the counter ion is negative and can be represented by "X"," e.g., as -N^ RwR^X" and -Z'- N+R13R14R15X". The counter ions for the positively charged substituents are anions
(specifically pharmaceutically acceptable anions), which include, but are not limited to, acetate, benzenesulfonate, benzoate, bicarbonate, bitartrate, bromide, calcium edetate, camsylate, carbonate, chloride, bromide, citrate, dihydrochloride, edetate, edisylate, estolate, esylate, fumarate, glyceptate, gluconate, glutamate, glycollylarsanilate, hexylresorcinate, hydroxynaphthoate, iodide, isethionate, lactate, lactobionate, malate, maleate, mandelate, mesylate, methylsulfate, mucate, napsylate, nitrate, pamoate, pantothenate,
phosphate/diphospate, polygalacturonate, salicylate, stearate, subacetate, succinate, sulfate, tannate, tartrate, teoclate, tosylate, and triethiodide. Specifically, the counter ions for the positively charged substituents are chloride, bromide, sulfate, and phosphate.
[64] The counter ions for the negatively charged substituents include, but are not limited to, an alkali metal ion (e.g. , sodium and potassium), an alkaline earth metal ion (e.g., calcium
and magnesium), aluminum ion, ammonium, protonated trialkyl amines (e.g. , trimethylamine and triethylamine), a tetraalkyl ammonium (e.g. , tetra methyl ammonium, and tetrabutyl ammonium), and a protonated heteroaromatic group (e.g. , pyridine, pyrimidine, triazines, tetrazines). Specifically, the counter ions for the negatively charged substituents are sodium, potassium, lithium, protonated triethyl amine, protonated pyridiene. Most specifically, the counter ions for the negatively charged substituents are sodium and potassium. The counter ions for both the negatively and positively charged substituents may be removed or replaced in subsequent purification steps.
[65] "Amino acid" as used herein, including the residue represented by variable "XX" described above, refers to naturally occurring amino acids, unnatural amino acids, amino acid analogs, or amino acid mimetics that function in a manner similar to the naturally occurring amino acids.
[66] The term "naturally occurring amino acids" as used herein refers to those twenty L- amino acids encoded by the universal genetic codes and appearing in proteins or peptides, as well as selenocysteine and pyrrolysine that are incorporated into proteins by distinctive biosynthetic mechanisms. They include histidine, alanine, isoleucine, arginine, leucine, asparagine, lysine, aspartic acid, methionine, cysteine, phenylalanine, glutamic acid, threonine, glutamine, tryptophan, glycine, valine, proline, serine, tyrosine, selenocysteine and pyrrolysine. The term "naturally occurring amino acids" also refers to those produced by the body, but are not encoded by the universal genetic codes, such as β-alanine, ornithine, and citrulline. The term "naturally occurring amino acids" further includes those naturally occurring L-amino acids that are later modified (e.g. , via post-translational modification by enzymes) in the body, such as hydroxyproline, γ-carboxyglutamate, and O-phospho serine.
[67] The term "unnatural amino acids" as used herein is intended to include the "D" stereochemical form of the naturally occurring amino acids described above. It is further understood that the term "unnatural amino acids" includes homologues of the natural L- amino acids or their D isomers, and synthetically modified forms of the natural L-amino acids or their D isomers. The synthetically modified forms include, but are not limited to, amino acids having side chains shortened or lengthened by up to two carbon atoms, amino acids comprising optionally substituted aryl groups, and amino acids comprised halogenated groups, specifically halogenated alkyl and aryl groups and also N substituted amino acids e.g. N-methyl-histidine, N-methyl-alanine, N-methyl-isoleucine, N-methyl-arginine, N-methyl- leucine, N-methyl-asparagine, N-methyl-lysine, N-methyl-aspartic acid, N-methyl- methionine, N-methyl-cysteine, N-methyl-phenylalanine, N-methyl-glutamic acid, N-methyl-
threonine, N-methyl-glutamine, N-methyl-tryptophan, N-methyl-glycine, N-methyl-valine, N-methyl-proline, N-methyl-serine, N-methyl-tyrosine, N-methyl-selenocysteine, and N- methyl-pyrrolysine, each including an L or D isomer. In one embodiment, the "unnatural amino acids" includes, for example, glutamic acid 5-methyl ester (Glu(OMe)), including an L or D isomer.
[68] The term "amino acid analogs" as used herein refers to compounds that have the same basic chemical structure as a naturally occurring amino acid, i.e., an a carbon that is bound to a hydrogen, a carboxyl group, an amino group, and an R group. They include 3- aminoalanine, 3-dimethylaminoalanine, 2-amino-4-(dimethylamino)butanoic acid, 2,4- diaminobutanoic acid, 2-amino-6-(dimethylamino)hexanoic acid, 2-amino-5- (dimethylamino)pentanoic acid, homoserine, norleucine, cysteine sulfonic acid, cysteine sulfinic acid, methionine sulfoxide, and methionine methyl sulfonium. Such analogs may have modified R groups (e.g. , norleucine) or modified peptide backbones, but retain the same basic chemical structure as a naturally occurring amino acid. Amino acid analogs also include D isomers of the above-referenced L-isomers.
[69] The term "amino acid mimetics" as used herein refers to chemical compounds that have a structure that is different from the general chemical structure of an amino acid, but functions in a manner similar to a naturally occurring amino acid.
[70] [XX]2-io denotes a peptide of 2 to 10 residues. Peptides are short polymers of amino acid monomers linked by peptide bonds, the covalent chemical bonds formed between two amino acid monomers when the carboxyl group of N-terminal monomer reacts with the amino group of the C-terminal monomer. A preferred peptide length is about two to ten amino acids as described above, although a peptide of longer length may also be used.
[71] Any one of the peptides described herein can be connected to the rest of the molecules in either direction. For example, when AA1, AA2, and AA3 each represent an amino acid (naturally-occurring, or unnatural), "AA1-AA2-AA3 in either direction" refers to the tripeptide N-AA1-AA2-AA3-C), and the tripeptide N-AA3-AA2-AA1-C). Unless otherwise specified, peptide sequences are represented conventionally, with the N-terminus to the left and the C-terminus to the right.
[72] In certain embodiments, [XX]1-10 is an amino acid represented by [XX] \ or a peptide represented by [XX]2-1o, in which each XX is the residue of an independently selected amino acid selected from the group consisting of: histidine, alanine, isoleucine, arginine, leucine, asparagine, lysine, aspartic acid, methionine, cysteine, phenylalanine, glutamic acid, threonine, glutamine, tryptophan, glycine, valine, proline, serine, tyrosine, selenocysteine,
and pyrrolysine, N-methyl-histidine, N-methyl-alanine, N-methyl-isoleucine, N-methyl- arginine, N-methyl-leucine, N-methyl-asparagine, N-methyl-lysine, N-methyl-aspartic acid, N-methyl-methionine, N-methyl-cysteine, N-methyl-phenylalanine, N-methyl-glutamic acid, N-methyl-threonine, N-methyl-glutamine, N-methyl-tryptophan, N-methyl-glycine, N- methyl-valine, N-methyl-proline, N-methyl-serine, N-methyl-tyrosine, N-methyl- selenocysteine, N-methyl-pyrrolysine, hydroxyproline, γ-carboxyglutamate, selinocysteine, O-phosphoserine, homoserine, norleucine, methionine sulfoxide, methionine methyl sulfonium, citrulline, ornithine, cysteine sulfonic acid, cysteine sulfinic acid, 3-aminoalanine, 3-dimethylaminoalanine, 2-amino-4-(dimethylamino)butanoic acid, 2,4-diaminobutanoic acid, 2-amino-6-(dimethylamino)hexanoic acid, 2-amino-5-(dimethylamino)pentanoic acid, and β-alanine, each independently as an L or D isomer. Each [XX] can additionally be glutamic acid 5-methyl ester (Glu(OMe)), including an L or D isomer. In another alternative, each XX is the residue of an independently selected amino acid selected from glycine or alanine, each independently as an L or D isomer.
[73] In certain embodiments, [XX] O is a peptide represented by [XX]2-5 (a peptide of 2 to 5 residues).
[74] In certain embodiments, the peptide represented by [XX]2_10 is cleavable by a protease. In one embodiment, the protease is a protease expressed in tumor tissue. In another embodiment, the protease is a lysosomal protease.
[75] The cleavable peptide refers to peptides containing a cleavage recognition sequence of a protease. A cleavage recognition sequence for a protease is an amino acid sequence recognized by the protease during proteolytic cleavage. Many protease cleavage sites are known in the art, and these and other cleavage sites can be included a linker, a spacer, or a linker moiety. See, e.g., Matayoshi et al., Science 247:954 (1990); Dunn et al., Meth.
Enzymol. 241:254 (1994); Seidah et al, Meth. Enzymol. 244: 175 (1994); Thornberry, Meth. Enzymol. 244:615 (1994); Weber et al., Meth. Enzymol. 244:595 (1994); Smith et al., Meth. Enzymol. 244:412 (1994); Bouvier et al., Meth. Enzymol. 248: 614(1995), Hardy et al, in AMYLOID PROTEIN PRECURSOR IN DEVELOPMENT, AGING, AND ALZHEIMER 'S DISEASE, Ed. Masters et al., pp. 190-198 (1994).
[76] In one embodiment, the peptide sequence is chosen based on its ability to be cleaved by a tumor-associated protease, e.g., a protease that is found on the surface of a cancerous cell or extracellularly in the vicinity of tumor cells. The examples of such proteases include thimet oligopeptidase (TOP), CDIO (neprilysin), a matrix metalloprotease (such as MMP2 or MMP9), a type II transmembrane serine protease (such as Hepsin, testisin, TMPRSS4 or
matriptase/MT-SPl), legumain and enzymes described in the following reference: Current Topics in Developmental Biology: Cell Surface Proteases, vol. 54 Zucker S. 2003, Boston, MA. The ability of a peptide to be cleaved by tumor-associated protease can be tested using in vitro protease cleavage assays known in the art.
[77] In another embodiment, the peptide sequence is chosen based on its ability to be cleaved by a lysosomal protease, which include cathepsins B, C, D, H, L and S, and furin. Specifically, the peptide sequence is capable of being cleaved by an appropriate isolated protease in vitro, which can be tested using in vitro protease cleavage assays known in the art.
[78] In certain embodiments, the peptide is selected from the group consisting of Val-Cit, Val-Lys, Phe-Lys, Lys-Lys, Ala-Lys, Phe-Cit, Leu-Cit, Lle-Cit, Trp, Cit, Phe-Ala, Phe-N9- tosyl-Arg, Phe-N9-nitro-Arg, Phe-Phe-Lys, D-Phe-Phe-Lys, Gly-Phe-Lys, Leu-Ala-Leu, Ile- Ala-Leu, Val-Ala-Val, Ala-Leu- Ala-Leu, β-Ala-Leu- Ala-Leu, Gly-Phe-Leu-Gly, Val-Arg, Arg-Val, Arg-Arg, Val-D-Cit, Val-D-Lys, Val-D-Arg, D- Val-Cit, D-Val-Lys, D-Val-Arg, D- Val-D-Cit, D- Val-D-Lys, D-Val-D-Arg, D-Arg-D-Arg, Ala- Ala, Ala-D-Ala, D-Ala-Ala, and D-Ala-D-Ala, Gly-Gly-Gly, Ala- Ala- Ala, D- Ala- Ala- Ala, Ala-D- Ala-Ala, Ala-Ala-D-Ala, Ala- Val-Cit, and Ala-Val-Ala. In another alternative, the peptide is selected from the group consisting of Gly-Gly-Gly, Ala- Ala- Ala, D- Ala- Ala- Ala, Ala-D- Ala- Ala, and Ala-Val-Ala. Alternatively, the peptide is Gly-Gly-Ala, Val-Ala, Glu-Ala, or Glu(OMe)-Ala. In a related embodiment, any of the peptide sequences herein above may be in either direction, as defined above.
[79] An amino acid or peptide can be attached to or be present in a linker or in a spacer, or be attached to a cell binding agent through the terminal amine or terminal carboxylic acid of the amino acid or peptide. The amino acid can also be attached to a linker or a spacer, or a cell-binding agent through a side chain reactive group, including, but not restricted to, the thiol group of cysteine, the epsilon amine of lysine, and the side chain hydroxyls of serine or threonine.
[80] The term "spacer" as used herein includes a chemical moiety interposed between any two chemical groups. For example, in some embodiments, one end of the spacer is linked to a cell-bind agent (e.g., an antibody, such as a human or humanized monoclonal antibody, or an antigen-binding portion or fragment thereof), or a reactive functional group that can form a covalent bond with a cell-binding agent. In other embodiments, one end of the spacer is linked to a cytotoxic drug (e.g., a maytansinoid, such as DM1 or DM4), or a reactive functional group that can form a covalent bond with a cytotoxic drug. In some embodiments, one end of the spacer is linked to a branched scaffold. In some embodiments, the spacer is
interposed between (1) a cell-binding agent, or a reactive functional group that can form a covalent bond with a cell-binding agent; and (2) a branched scaffold. In some embodiments, the spacer is interposed between (1) a cytotoxic drug, or a reactive functional group that can form a covalent bond with a cytotoxic drug; and (2) a branched scaffold. A spacer may be attached to a reactive functional group at one end to form a linker moiety that can further react with a cell-binding agent or a cytotoxic drug.
[81] In certain embodiments, the spacer creates a desired distance between the two chemical groups to, for example, avoid stereo hindrance or to promote molecular flexibility. In certain embodiments, the presence of the spacer does not hinder, inhibit, or otherwise negatively affect the function of the flanking chemical groups (e.g., the ability of the cell- binding agent to bind a target molecule on a cell, or the cytotoxicity of the cytotoxic drug). In certain embodiments, the spacer confers additional beneficial characteristics, such as enhanced potency, solubility, serum stability, and/or efficacy, to the immunoconjugate or linker compound comprising the spacer. In certain embodiments, the spacer may comprise one or more amino acid residues (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more residues), which may or may not be resistant to protease or peptidases (such as intracellular / lysosomal peptidase) cleavage. In certain embodiments, the spacer may comprise one or more repeats of polyethylene glycol (PEG) units -(CH2-CH2-0)-, such as 1-1000 PEG units, 1-500 PEG units, 1-24 PEG units, or 2-8 PEG units (2, 4, 6, or 8 PEG units). Exemplary spacers hi and L2 are described in more details below.
[82] The term "branched scaffold" as used herein includes a chemical moiety linked to three or more spacers. A branched scaffold allows two or more drugs to be attached to the cell binding agent. Exemplary branched scaffolds may be derived from an amino acid with a side chain comprising an amino group (such as Lys) or a carboxyl group (such as a Glu or an Asp), or a peptide comprising two or more such amino acids (e.g., Lys-Lys dimer etc.). Each amino acid may be independently D- or L-amino acids. Chemically modified amino acids or analogs thereof having similar structures may also give rise to branches scaffolds. In certain embodiments, one cell-binding agent and two or more cytotoxic drugs (same or different) may be linked to the branched scaffold, each independently through a spacer (same or different).
[83] The term "residue of a functional group" is a moiety remaining after a reaction with another functional group. For example, a residue of thiol group can form disulfide bond after reacting with another thiol group or thioether after reacting with an electrophilic group, such as maleimide.
[84] The term "reactive ester" as used herein refers to an ester group having a leaving group that is readily displaced by an amine group. Examples of a reactive ester, include, but are not limited to, N-hydroxysuccinimide ester, N-hydroxysulfosuccinimide ester, nitrophenyl (e.g., 2 or 4-nitrophenyl) ester, dinitrophenyl (e.g., 2,4-dinitrophenyl) ester, sulfo- tetraflurophenyl (e.g., 4-sulfo-2,3,5,6-tetrafluorophenyl)ester and pentafluorophenyl ester.
[85] As used herein, the terms "DARPin" and "(designed) ankyrin repeat protein" are used interchangeably to refer to certain genetically engineered antibody mimetic proteins typically exhibiting preferential (sometimes specific) target binding. The target may be protein, carbohydrate, or other chemical entities, and the binding affinity can be quite high. The DARPins may be derived from natural ankyrin repeat-containing proteins, and specifically consist of at least three, usually four or five ankyrin repeat motifs (typically about 33 residues in each ankyrin repeat motif) of these proteins. In certain embodiments, a DARPin contains about four- or five-repeats, and may have a molecular mass of about 14 or 18 kDa, respectively. Libraries of DARPins with randomized potential target interaction residues with diversities of over 10 12 variants can be generated at the DNA level, for use in selecting DARPins that bind desired targets (e.g., acting as receptor agonists or antagonists, inverse agonists, enzyme inhibitors, or simple target protein binders) with picomolar affinity and specificity, using a variety of technologies such as ribosome display or signal recognition particle (SRP) phage display. See, for example, U.S. Patent Publication Nos. 2004/0132028, 2009/0082274, 2011/0118146, and 2011/0224100, WO 02/20565 and WO 06/083275(the entire teachings of which are incorporated herein by reference), and also see C. Zahnd et ah, Cancer Res. (2010) 70: 1595-1605; Zahnd et al, J. Biol. Chem. (2006) 281(46):35167-35175; and Binz, H.K., Amstutz, P. & Pluckthun, A., Nature Biotechnology (2005) 23: 1257-1268 (all incorporated herein by reference). Also see U.S. Patent Publication No. 2007/0238667; U.S. Patent No. 7,101,675; WO 2007/147213; and WO 2007/062466 (the entire teachings of which are incorporated herein by reference), for the related ankyrin-like repeats protein or synthetic peptide. As used herein, "AVIBODY™" cell binding agents (or "AVIBODY CBA" in short) include a family of proteins as cell binding agents that specifically bind desired targets. As is well known, antibodies bind such desired targets through "Target Binding Regions" (TBRs) or Fv domains. AVIBODY™ CBA typically contains two, three, or four TBRs more commonly known as Dia-, Tria- and Tetra-bodies. These TBRs / Fv domains are linked together by fusing the Fv V-domains together in a "head to tail" orientation, forming stable, specific, and highly customizable multimeric antibody-like
proteins as AVIBODY™ CBA. See, for example, U.S. Publication Nos. 2008/0152586 and 2012/0171115 for details, the entire teachings of which are incorporated herein by reference.
[86] The term "cell binding agent" as used herein refers to a compound that can bind a cell (e.g., on a cell-surface ligand) or bind a ligand associated with or proximate to the cell, either in a specific or non-specific manner. In certain embodiments, binding to the cell or a ligand on or near the cell is specific. The cell-binding agent may be of any kind presently known, or that become known and includes peptides and non-peptides.
[87] In certain embodiments, the cell-binding agents are proteins or polypeptides, or compounds comprising proteins or polypeptides, including antibody and non-antibody proteins or polypeptides. Specifically, the cell-binding agents (e.g., proteins or polypeptides) comprise one or more Cys residues. The side chain -SH group of the Cys residues may be intact, or may be in a disulfide bond that can be reduced. Specifically, reduction of the disulfide bond(s) does not significantly negatively impact the cell-binding function of the proteins or polypeptides (e.g., in the case of antibody or antigen-binding portion thereof, reduction of the disulfide bonds does not substantially increase the dissociation of light chains / heavy chains). Alternatively or in addition, a cell-binding agent (e.g., a protein such as an antibody, or polypeptide) may be a part of a modified cell-binding agent, which is linked to a spacer connected to a branched scaffold, which branched scaffold is capable of being connected to two or more cytotoxic drugs, each through a reactive functional group attached to a spacer linked to the branched scaffold. An exemplary such modified cell- binding agent is represented in Formula (IV). The reactive functional group can be -SH, -C(=0)-, -NHNH2, -N3, -alkyne, tetrazine, strained cycloalkene or strained
heterocycloalkene, dithioester, or diene (see, for example, Vu Hong et al, Bioconjugate Chem. (2010) 21(10): 1912-1916; Glassner, M. et al., J. Am. Chem. Soc. (2012) 134:7274- 7277; Hansell, C. F. et al, J. Am. Chem. Soc. (2011) 133: 13828-13831; Neal K. Devaraj, Synlett (2012) 23(15):2147-2152; Chenoweth, K. et al, Organic & Biomolecular Chemistry (2009) (24):5255; Jewett, J.C. et al, J. Am. Chem. Soc. (2010) 132(11):3688-3690; Seitchik J.L. et al, J. Am. Chem. Soc. (2012) 134(6):2898-2901; and Sletten E.M. et al, Angew Chem. Int. Ed. Engl.( 2009) 48(38):6974-6998). The reactive functional group can be introduced into the modified cell-binding agent through any chemical or enzymatic method known in the art. See, for example, Davis L.K. et al., /. Am. Chem. Soc. (2012) 134: 10317- 10320; Boeggeman E, et al., Bioconjug Chem. (2009 Jun 20) (6): 1228-36; Stan AC, et al, Cancer Res. (1999 Jan 1) 59(1): 115-21 ; Mahal, L. K. et al, Science (1997) 276: 1125-1128; Saxon, E., and Bertozzi, C. R., Science (2000) 287:2007-2010; Hang, H. C. et al, Proc. Natl.
Acad. Sci. USA (2003) 100: 14846-14851; Vocadlo, D. J. et al, Proc. Natl. Acad. Sci. USA (2003) 100:9116-9121; Prescher, J. A. et al., Nature (2004) 430:873-877; Dube, D. H. et al., Proc. Natl. Acad. Sci. USA (2006) 103:4819-4824; Jeger S, et al, Angew Chem Int Ed Engl. (2010 Dec 17) 49(51):9995-9997; and Lang K. et al, J. Am. Chem. Soc. (2012) 134: 10317- 10320.
[88] Cell-binding agent can also be peptides derived from phage display (see, for example, Wang et al, Proc. Natl. Acad. Sci. USA (2011) 108(17):6909-6914) or peptide library techniques (see, for example, Dane et al., Mol. Cancer. Ther. (2009) 8(5): 1312-1318).
[89] In one embodiment, one or more reactive functional groups that are capable of reacting with the cytotoxic drug can be introduced into the modified cell-binding agent (e.g., Formula (IV)) by any methods known in the art. For example, a terminal amine group on the modified cell-binding agent can be converted to a carbonyl group through transamination reaction (see, for example, US 2010/0099649; Angew. Chem. Int. Ed., 45(32):5307 (2006); Chem. Biol., 2(4):247 (2007); /. Am. Chem. Soc, 130(35): 11762, 2008).
[90] The cell-binding agent may be linked to a spacer through a free Cys residue, which can be engineered into the cell-binding agent if necessary (i.e., cysteine residues having a free -SH group that can react with a reactive functional group attached to a spacer) according to any methods known in the art (see, for example, US 7,521,541). In another alternative, thiol groups (-SH) can be generated by controlled reduction of interchain disulfides of antibodies, followed by treatment with a maleimido group as the reactive functional group, as described in US patent 7,659,241. Thiol groups can also be introduced into the cell-binding agent (e.g., antibodies) by reaction with a crosslinking agent such as 2-iminothiolane (see for example Goff and Carroll, Bioconjugate Chem. (1990) 1(6):381— 386) followed by reaction with a maleimido group to form a modified cell-binding agent. All these methods for introducing reactive functional groups are applicable of being used for cell-binding agents that are not antibodies, which, for example, include Centyrin, DARPin, Avibody, adnectin or antibody fragment, such as minibodies, diabodies, tribodies, tetrabodies, nanobodies, probodies, domain bodies or unibodies.
[91] In one embodiment, when the cell-binding agent is a Centyrin, one or more reactive functional groups (e.g., a cysteine having a free thiol group) can be introduced according to methods described in US 2010/0255056, US 2010/0216708 and US 2011/0274623.
[92] In another embodiment, the cell-binding agent is a DARPin and it can be prepared according to methods described in US Publication Nos. 2004/0132028, 2009/0082274,
2011/0118146, and 2011/0224100, WO 02/20565 and WO 06/083275. Specifically, DARPin
comprises one or more cysteine residues at specific positions that do not interfere with antigen binding. Such cysteine residue can react with the reactive functional groups attached to a spacer.
[93] In yet another embodiment, Avibodies having one or more cysteine residues can be prepared according to methods described in US 2008/0139791 and US 2012/0171115.
[94] The Cys side chain -SH groups may react with a reactive functional group described above covalently linked to a spacer connected to the branched scaffold, which can in turn be linked to two or more cytotoxic compounds through additional reactive functional groups attached to spacers, thus conjugating the cell-binding agents to the cytotoxic compounds to yield the conjugates of the invention (e.g., conjugates of Formula (I)). Each protein-based cell-binding agents may contain multiple Cys side chain -SH groups, each available for linking the cell-binding agent to a spacer.
[95] Examples of the cell binding agents include an antibody, a single chain antibody, an antibody fragment that specifically binds to the target cell, a monoclonal antibody, a single chain monoclonal antibody, a monoclonal antibody fragment that specifically binds to a target cell, a chimeric antibody, a chimeric antibody fragment that specifically binds to the target cell, a bispecific antibody, a domain antibody, a domain antibody fragment that specifically binds to the target cell, an interferon, a lymphokine (e.g., IL-2, IL-3, IL-4, and IL-6), a hormone (e.g., insulin, thyrotropin releasing hormone, melanocyte- stimulating hormone, and a steroid hormone (e.g., androgen and estrogen)), a vitamin (e.g., folate), a growth factor (e.g., EGF, TGF-alpha, FGF, VEGF), a colony stimulating factor, a nutrient- transport molecule (e.g., transferrin; see O'Keefe et al., J. Biol. Chem. (1985) 260:932-937, incorporated herein by reference), a Centyrin (a protein scaffold based on a consensus sequence of fibronectin type III (FN3) repeats; see U.S. Patent Publication Nos.
2010/0255056, 2010/0216708 and 2011/0274623 incorporated herein by reference), an Ankyrin Repeat Protein (e.g., a designed ankyrin repeat protein, known as DARPin; see U.S. Patent Publication Nos. 2004/0132028, 2009/0082274, 2011/0118146, and 2011/0224100, incorporated herein by reference, and also see C. Zahnd et al., Cancer Res. (2010) 70: 1595- 1605; Zahnd et al, J. Biol. Chem. (2006) 281(46):35167-35175; and Binz, H.K., Amstutz, P. & Pluckthun, A., Nature Biotechnology (2005) 23: 1257-1268, incorporated herein by reference), an ankyrin-like repeats protein or synthetic peptide (see e.g., U.S. Patent
Publication No. 2007/0238667; U.S. Patent No. 7,101,675; WO 2007/147213; and
WO 2007/062466, incorporated herein by reference), an Adnectin (a fibronectin domain scaffold protein; see US Patent Publication Nos. 2007/0082365; 2008/0139791, incorporated
herein by reference), Avibody (including diabodies, triabodies, and tetrabodies; see U.S. Publication Nos. 2008/0152586 and 2012/0171115), and other cell-binding molecules or substances.
[96] In certain embodiments, the cell-binding agent is an antibody, a single chain antibody, an antibody fragment that specifically binds to the target cell, a monoclonal antibody, a single chain monoclonal antibody, a monoclonal antibody fragment that specifically binds to a target cell, a chimeric antibody, a chimeric antibody fragment that specifically binds to the target cell, a domain antibody, a domain antibody fragment that specifically binds to the target cell, a lymphokine, a hormone, a vitamin, a growth factor, a colony stimulating factor, or a nutrient-transport molecule. Alternatively, the cell-binding agent is a monoclonal antibody, a single chain monoclonal antibody, or a monoclonal antibody fragment that specifically binds to a target cell.
[97] In certain embodiments, the cell-binding agent is a bispecific antibody, an ankyrin repeat protein, a Centyrin, or an Avibody.
[98] "Antibody fragment" and "antigen-binding portion or fragment" are used interchangeably here to refer to Fab, Fab', and F(ab')2, Fv, minibodies, diabodies, tribodies, tetrabodies, nanobodies, probodies, domain bodies, unibodies, and the like (Parham, J.
Immunol. 131:2895-2902 (1983); Spring et al, J. Immunol. 113:470-478 (1974); Nisonoff et al, Arch. Biochem. Biophys. 89:230-244 (1960), Kim et al, Mol. Cancer Ther., 7:2486-2497 (2008), Carter, Nature Revs., 6:343-357 (2006), R. Kontermann & S. Dubel, 2001 Antibody Engineering, Springer- Verlag, Heidelberg-New York).
[99] In certain embodiments, the cell-binding agent is a minibody, a diabody, a tribody, a tetrabody, a nanobody, a probody, a domain body, or an unibody.
[100] Monoclonal antibody techniques allow for the production of extremely specific cell- binding agents in the form of specific monoclonal antibodies. Particularly well known in the art are techniques for creating monoclonal antibodies produced by immunizing mice, rats, hamsters or any other mammal with the antigen of interest such as the intact target cell, antigens isolated from the target cell, whole virus, attenuated whole virus, and viral proteins such as viral coat proteins. Sensitized human cells can also be used. Another method of creating monoclonal antibodies is the use of phage libraries of scFv (single chain variable region), specifically human scFv {see e.g., Griffiths et al, U.S. Patent Nos. 5,885,793 and 5,969,108; McCafferty et al, WO 92/01047; Liming et al, WO 99/06587). In addition, resurfaced antibodies disclosed in U.S. Patent No. 5,639,641 may also be used, as may chimeric antibodies and humanized antibodies.
[101] Selection of the appropriate cell-binding agent is a matter of choice that depends upon the particular cell population that is to be targeted, but in general human monoclonal antibodies are preferred if an appropriate one is available. For example, the monoclonal antibody MY9 is a murine IgGi antibody that binds specifically to the CD33 Antigen (J.D. Griffin et ah, Leukemia Res. (1984) 8:521) and can be used if the target cells express CD33 as in the disease of acute myelogenous leukemia (AML).
[102] In one embodiment, the cell-binding agent is a resurfaced antibody, a resurfaced single chain antibody, or a resurfaced antibody fragment.
[103] In another embodiment, the cell-binding agent is a humanized antibody, a humanized single chain antibody, or a humanized antibody fragment. In a specific embodiment, the humanized antibody is huMy9-6 or another related antibody, which is described in U.S. Pat. Nos. 7,342,110 and 7,557, 189. In another specific embodiment, the humanized antibody is an anti-folate receptor antibody described in U.S. Provisional Application Nos. 61/307,797, 61/346,595, and 61/413,172 and U.S. Application No. 13/033,723 (published as US
2012/0009181 Al). The teachings of all these applications are incorporated herein by reference in its entirety.
[104] In certain embodiments, the cell-binding agent is an antigen -binding portion of a monoclonal antibody, sharing sequences critical for antigen-binding with an antibody disclosed herein, such as huMy9-6 or its related antibodies described in U.S. Pat. Nos.
7,342, 110 and 7,557, 189, incorporated herein by reference. These derivative antibodies may have substantially the same or identical (1) light chain and/or heavy chain CDR3 regions; (2) light chain and/or heavy chain CDR1, CDR2, and CDR3 regions; or (3) light chain and/or heavy chain regions, compared to an antibody described herein. Sequences within these regions may contain conservative amino acid substitutions, including substitutions within the CDR regions. Specifically, there is no more than 1, 2, 3, 4, or 5 conservative substitutions. In an alternative, the derivative antibodies have a light chain region and/or a heavy chain region that is at least about 90%, 95%, 99% or 100% identical to an antibody described herein. These derivative antibodies may have substantially the same binding specificity and/or affinity to the target antigen compared to an antibody described herein. Specifically, the Kd and/or R values of the derivative antibodies are within 10-fold (either higher or lower), 5-fold (either higher or lower), 3-fold (either higher or lower), or 2-fold (either higher or lower) of an antibody described herein. These derivative antibodies may be fully human antibodies, or humanized antibodies, or chimeric antibodies. The derivative antibodies may be produced according to any art-recognized methods.
[105] Specific exemplary antigens or ligands include renin; a growth hormone (e.g. , human growth hormone and bovine growth hormone); a growth hormone releasing factor; a parathyroid hormone; a thyroid stimulating hormone; a lipoprotein; alpha- 1- antitrypsin; insulin A-chain; insulin B-chain; proinsulin; a follicle stimulating hormone; calcitonin; a luteinizing hormone; glucagon; a clotting factor (e.g. , factor vmc, factor IX, tissue factor, and von Willebrands factor); an anti-clotting factor (e.g., Protein C); an atrial natriuretic factor; a lung surfactant; a plasminogen activator (e.g., a urokinase, a human urine or tissue-type plasminogen activator); bombesin; a thrombin; hemopoietic growth factor; tumor necrosis factor- alpha and -beta; an enkephalinase; RANTES (i.e., the regulated on activation normally T-cell expressed and secreted); human macrophage inflammatory protein- 1 -alpha; a serum albumin (human serum albumin); Muellerian-inhibiting substance; relaxin A-chain; relaxin B-chain; prorelaxin; a mouse gonadotropin-associated peptide; a microbial protein (beta- lactamase); DNase; IgE; a cytotoxic T-lymphocyte associated antigen (e.g. , CTLA-4);
inhibin; activin; a vascular endothelial growth factor; protein A or D; a rheumatoid factor; a neurotrophic factor(e.g. , bone-derived neurotrophic factor, neurotrophin-3, -4, -5, or -6), a nerve growth factor (e.g. , NGF-β); a platelet-derived growth factor; a fibroblast growth factor (e.g., aFGF and bFGF); fibroblast growth factor receptor 2; an epidermal growth factor; a transforming growth factor (e.g., TGF-alpha, TGF-βΙ, TGF- 2, TGF- 3, TGF- 4, and TGF- β5); insulin-like growth factor-I and -II; des(l-3)-IGF-I (brain IGF-I); an insulin-like growth factor binding protein; melanotransferrin; EpCAM; GD3; FLT3; PSMA; PSCA; MUC1 ; MUC16; STEAP; CEA; TENB2; an EphA receptor; an EphB receptor; a folate receptor; FOLR1 ; mesothelin; cripto; an alphavbeta6; integrins; VEGF; VEGFR; EGFR; transferrin receptor; IRTAl ; IRTA2; IRTA3; IRTA4; IRTA5; CD proteins (e.g., CD2, CD3, CD4, CD5, CD6, CD8, CD11, CD14, CD19, CD20, CD21, CD22, CD25, CD26, CD28, CD30, CD33, CD36, CD37, CD38, CD40, CD44, CD52, CD55, CD56, CD59, CD70, CD79, CD80. CD81, CD103, CD105, CD134, CD137, CD138, and CD152), one or more tumor- associated antigens or cell-surface receptors (see US Publication No. 20080171040 or US Publication No. 20080305044, incorporated in their entirety by reference); erythropoietin; an
osteoinductive factor; an immunotoxin; a bone morphogenetic protein; an interferon (e.g. , interferon-alpha, -beta, and -gamma); a colony stimulating factor (e.g., M-CSF, GM-CSF, and G-CSF); interleukins (e.g., IL- 1 to IL- 10); a superoxide dismutase; a T-cell receptor; a surface membrane protein; a decay accelerating factor; a viral antigen s,(e.g. , a portion of the HIV envelope); a transport protein, a homing receptor; an addressin; a regulatory protein; an integrin (e.g., CDl la, CDl lb, CDl lc, CD18, an ICAM, VLA-4, and VCAM;) a tumor
associated antigen (e.g. , HER2, HER3 and HER4 receptor); endoglin; c-Met; c-kit; 1GF1R; PSGR; NGEP; PSMA; PSCA; TMEFF2; LGR5; B7H4; and fragments of any of the above- listed polypeptides.
[106] For example, GM-CSF, a ligand / growth factor which binds to myeloid cells can be used as a cell-binding agent to diseased cells from acute myelogenous leukemia. IL-2 which binds to activated T-cells can be used for prevention of transplant graft rejection, for therapy and prevention of graft-versus-host disease, and for treatment of acute T-cell leukemia.
MSH, which binds to melanocytes, can be used for the treatment of melanoma, as can antibodies directed towards melanomas. Folic acid can be used to target the folate receptor expressed on ovarian and other tumors. Epidermal growth factor can be used to target squamous cancers, such as lung and head and neck. Somatostatin can be used to target neuroblastomas and other tumor types. Estrogen (or estrogen analogues) can be used to target breast cancer. Androgen (or androgen analogues) can be used to target testes.
[107] The term "salt" as used herein refers to organic or inorganic salts of a compound of the invention. Specifically, a salt is a pharmaceutically acceptable salt. Other non- pharmaceutically acceptable salts are also included in the present invention. The salts include salts, formed by reacting a compound of the invention, which comprises a basic group, with an inorganic acid or organic acid (such as a carboxylic acid), and salts, formed by reacting a compound of the invention, which comprises an acidic group, with an inorganic base or organic base (such as an amine). Exemplary salts include those pharmaceutically acceptable salts described immediately below.
[108] The term "pharmaceutically acceptable salt" as used herein refers to
pharmaceutically acceptable organic or inorganic salts of a compound of the invention.
Exemplary salts include, but are not limited, to sulfate, citrate, acetate, oxalate, chloride, bromide, iodide, nitrate, bisulfate, phosphate, acid phosphate, isonicotinate, lactate, salicylate, acid citrate, tartrate, oleate, tannate, pantothenate, bitartrate, ascorbate, succinate, maleate, gentisinate, fumarate, gluconate, glucuronate, saccharate, formate, benzoate, glutamate, methanesulfonate "mesylate," ethanesulfonate, benzenesulfonate, p-toluenesulfonate, pamoate (i.e. , l,l '-methylene-bis-(2-hydroxy-3-naphthoate)) salts, alkali metal (e.g., sodium and potassium) salts, alkaline earth metal (e.g., magnesium) salts, and ammonium salts. A pharmaceutically acceptable salt may involve the inclusion of another molecule such as an acetate ion, a succinate ion or other counter ion. The counter ion may be any organic or inorganic moiety that stabilizes the charge on the parent compound. Furthermore, a pharmaceutically acceptable salt may have more than one charged atom in its structure.
Instances where multiple charged atoms are part of the pharmaceutically acceptable salt can have multiple counter ions. Hence, a pharmaceutically acceptable salt can have one or more charged atoms and/or one or more counter ion.
[109] If the compound of the invention contains one or more basic moieties, desired salts (e.g., pharmaceutically acceptable salts) may be prepared by any suitable method available in the art, for example, treatment of the free base with an inorganic acid, such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, methanesulfonic acid, phosphoric acid and the like, or with an organic acid, such as acetic acid, maleic acid, succinic acid, mandelic acid, fumaric acid, malonic acid, pyruvic acid, oxalic acid, glycolic acid, salicylic acid, a pyranosidyl acid, such as glucuronic acid or galacturonic acid, an alpha hydroxy acid, such as citric acid or tartaric acid, an amino acid, such as aspartic acid or glutamic acid, an aromatic acid, such as benzoic acid or cinnamic acid, a sulfonic acid, such as p-toluenesulfonic acid or ethanesulfonic acid, or the like.
[110] If the compound of the invention contains one or more acidic moieties, desired salts (e.g., pharmaceutically acceptable salts) may be prepared by any suitable method, for example, treatment of the free acid with an inorganic, such as an alkali metal hydroxide or alkaline earth metal hydroxide, organic base, such as an amine (primary, secondary or tertiary), or the like. Illustrative examples of suitable salts include, but are not limited to, organic salts derived from amino acids, such as glycine and arginine, ammonia, primary, secondary, and tertiary amines, and cyclic amines, such as piperidine, morpholine and piperazine, and inorganic salts derived from sodium, calcium, potassium, magnesium, manganese, iron, copper, zinc, aluminum and lithium.
[I l l] The terms "abnormal cell growth" and "proliferative disorder" are used interchangeably in this application. "Abnormal cell growth," as used herein, unless otherwise indicated, refers to cell growth that is independent of normal regulatory
mechanisms (e.g. , loss of contact inhibition). This includes, for example, the abnormal growth of: (1) tumor cells (tumors) that proliferate by expressing a mutated tyrosine kinase or overexpression of a receptor tyrosine kinase; (2) benign and malignant cells of other proliferative diseases in which aberrant tyrosine kinase activation occurs; (3) any tumors that proliferate by receptor tyrosine kinases; (4) any tumors that proliferate by aberrant serine/threonine kinase activation; and (5) benign and malignant cells of other proliferative diseases in which aberrant serine/threonine kinase activation occurs.
[112] The terms "cancer" and "cancerous" refer to or describe the physiological condition in mammals that is typically characterized by unregulated cell growth. A "tumor" comprises
one or more cancerous cells, and/or benign or pre-cancerous cells. Examples of cancer include, but are not limited to, carcinoma, lymphoma, blastoma, sarcoma, and leukemia or lymphoid malignancies. More particular examples of such cancers include skin cancer (e.g., melanoma), Merkel cell carcinoma, squamous cell cancer (e.g. , epithelial squamous cell cancer), lung cancer (e.g. , small-cell lung cancer, non-small cell lung cancer, adenocarcinoma of the lung, and squamous carcinoma of the lung), cancer of the peritoneum, hepatocellular cancer, gastric or stomach cancer (e.g. , gastrointestinal cancer), pancreatic cancer, cervical cancer, ovarian cancer, liver cancer, bladder cancer, hepatoma, breast cancer, colon cancer, rectal cancer, colorectal cancer, endometrial or uterine carcinoma, salivary gland carcinoma, kidney or renal cancer, prostate cancer, testicular cancer, vulval cancer, thyroid cancer, hepatic carcinoma, anal carcinoma, penile carcinoma, acute leukemia, head and neck cancer, brain cancer (e.g., glioblastoma and neuroblastoma), cancers of lymphatic organs and hematological malignancy including Leukemia (Acute lymphoblastic leukemia (ALL), Acute myelogenous leukemia (AML), Chronic lymphocytic leukemia (CLL), Chronic myelogenous leukemia (CML), Acute monocytic leukemia (AMOL), Hairy cell leukemia (HCL), T-cell prolymphocytic leukemia (T-PLL), Large granular lymphocytic leukemia, Adult T-cell leukemia), Lymphoma (small lymphocytic lymphoma (SLL), Hodgkin's lymphomas (Nodular sclerosis, Mixed cellularity, Lymphocyte-rich, Lymphocyte depleted or not depleted, and Nodular lymphocyte-predominant Hodgkin lymphoma), Non-Hodgkin's lymphomas (all subtypes), Chronic lymphocytic leukemia/Small lymphocytic lymphoma, B- cell prolymphocytic leukemia, Lymphoplasmacytic lymphoma (such as Waldenstrom macro globulinemia), Splenic marginal zone lymphoma, Plasma cell neoplasms (Plasma cell myeloma, Plasmacytoma, Monoclonal immunoglobulin deposition diseases, Heavy chain diseases), Extranodal marginal zone B cell lymphoma (MALT lymphoma), Nodal marginal zone B cell lymphoma (NMZL), Follicular lymphoma, Mantle cell lymphoma, Diffuse large B cell lymphoma, Mediastinal (thymic) large B cell lymphoma, Intravascular large B cell lymphoma, Primary effusion lymphoma, Burkitt lymphoma/leukemia, T cell prolymphocytic leukemia, T cell large granular lymphocytic leukemia, Aggressive NK cell leukemia, Adult T cell leukemia/lymphoma, Extranodal NK/T cell lymphoma (nasal type), Enteropathy-type T cell lymphoma, Hepatosplenic T cell lymphoma, Blastic NK cell lymphoma, Mycosis fungoides / Sezary syndrome, Primary cutaneous CD30-positive T cell lymphoproliferative disorders, Primary cutaneous anaplastic large cell lymphoma, Lymphomatoid papulosis, Angioimmunoblastic T cell lymphoma, Peripheral T cell lymphoma (unspecified), Anaplastic large cell lymphoma), multiple myeloma (plasma cell myeloma or Kahler's disease).
[113] The term "therapeutic agent" encompasses both a biological agent such as an antibody, a peptide, a protein, an enzyme or a chemotherapeutic agent.
[114] The "cytotoxic compound" is referred to as "D" or "DM" interchangeably.
[115] The term "chemotherapeutic agent" or "cytotoxic drug" includes is a chemical compound useful in the treatment of cancer. Examples of chemotherapeutic agents include Erlotinib (TARCEVA®, Genentech/OSI Pharm.), Bortezomib (VELCADE®, Millennium Pharm.), Fulvestrant (FASLODEX®, AstraZeneca), Sutent (SU11248, Pfizer), Letrozole (FEMARA®, Novartis), Imatinib mesylate (GLEEVEC®, Novartis), PTK787/ZK 222584 (Novartis), Oxaliplatin (Eloxatin®, Sanofi), 5-FU (5-fluorouracil), Leucovorin, Rapamycin (Sirolimus, RAPAMUNE®, Wyeth), Lapatinib (TYKERB®, GSK572016, Glaxo Smith Kline), Lonafarnib (SCH 66336), Sorafenib (BAY43-9006, Bayer Labs), and Gefitinib (IRESSA®, AstraZeneca), AG1478, AG1571 (SU 5271; Sugen), alkylating agents such as thiotepa and CYTOXAN® cyclosphosphamide; alkyl sulfonates such as busulfan, improsulfan and piposulfan; aziridines such as benzodopa, carboquone, meturedopa, and uredopa; ethylenimines and methylamelamines including altretamine, triethylenemelamine, triethylenephosphoramide, triethylenethiophosphoramide and trimethylomelamine;
acetogenins (especially bullatacin and bullatacinone); a camptothecin (including the synthetic analog topotecan); bryostatin; callystatin; CC-1065 (including its adozelesin, carzelesin and bizelesin synthetic analogs); cryptophycins (particularly cryptophycin 1 and cryptophycin 8); dolastatin; duocarmycin (including the synthetic analogs, KW-2189 and CB1-TM1);
eleutherobin; pancratistatin; a sarcodictyin; spongistatin; nitrogen mustards such as chlorambucil, chlornaphazine, chlorophosphamide, estramustine, ifosfamide,
mechlorethamine, mechlorethamine oxide hydrochloride, melphalan, novembichin, phenesterine, prednimustine, trofosfamide, uracil mustard; nitrosureas such as carmustine, chlorozotocin, fotemustine, lomustine, nimustine, and ranimnustine; antibiotics such as the enediyne antibiotics (e.g., calicheamicin, especially calicheamicin gammall and
calicheamicin omegall (Angew Chem. Intl. Ed. Engl. (1994) 33: 183-186); dynemicin, including dynemicin A; bisphosphonates, such as clodronate; an esperamicin; as well as neocarzino statin chromophore and related chromoprotein enediyne antibiotic chromophores), aclacinomysins, actinomycin, authramycin, azaserine, bleomycins, cactinomycin, carabicin, caminomycin, carzinophilin, chromomycinis, dactinomycin, daunorubicin, detorubicin, 6- diazo-5-oxo-L-norleucine, ADRIAMYCIN® (doxorubicin), morpholino-doxorubicin, cyanomorpholino-doxorubicin, 2-pyrrolino-doxorubicin and deoxydoxorubicin), epirubicin, esorubicin, idarubicin, marcellomycin, mitomycins such as mitomycin C, mycophenolic acid,
nogalamycin, olivomycins, peplomycin, porfiromycin, puromycin, quelamycin, rodorubicin, streptonigrin, streptozocin, tubercidin, ubenimex, zinostatin, zorubicin; anti-metabolites such as methotrexate and 5-fluorouracil (5-FU); folic acid analogs such as denopterin,
methotrexate, pteropterin, trimetrexate; purine analogs such as fludarabine, 6- mercaptopurine, thiamniprine, thioguanine; pyrimidine analogs such as ancitabine, azacitidine, 6-azauridine, carmofur, cytarabine, dideoxyuridine, doxifluridine, enocitabine, floxuridine; androgens such as calusterone, dromostanolone propionate, epitiostanol, mepitiostane, testolactone; anti-adrenals such as aminoglutethimide, mitotane, trilostane; folic acid replenisher such as frolinic acid; aceglatone; aldophosphamide glycoside;
aminolevulinic acid; eniluracil; amsacrine; bestrabucil; bisantrene; edatraxate; defofamine; demecolcine; diaziquone; elformithine; elliptinium acetate; an epothilone; etoglucid; gallium nitrate; hydroxyurea; lentinan; lonidainine; maytansinoids such as maytansine and ansamitocins; mitoguazone; mitoxantrone; mopidanmol; nitraerine; pentostatin; phenamet; pirarubicin; losoxantrone; podophyllinic acid; 2-ethylhydrazide; procarbazine; PSK® polysaccharide complex (JHS Natural Products, Eugene, Oreg.); razoxane; rhizoxin;
sizofuran; spirogermanium; tenuazonic acid; triaziquone; 2,2' ,2"-trichlorotriethylamine; trichothecenes (especially T-2 toxin, verracurin A, roridin A and anguidine); urethan;
vindesine; dacarbazine; mannomustine; mitobronitol; mitolactol; pipobroman; gacytosine; arabinoside ("Ara-C"); cyclophosphamide; thiotepa; taxoids, e.g., TAXOL® (paclitaxel; Bristol-Myers Squibb Oncology, Princeton, N.J.), ABRAXANE® (Cremophor-free), albumin-engineered nanoparticle formulations of paclitaxel (American Pharmaceutical Partners, Schaumberg, 111.), and TAXOTERE® (doxetaxel; Rhone-Poulenc Rorer, Antony, France); chloranmbucil; GEMZAR® (gemcitabine); 6-thioguanine; mercaptopurine;
methotrexate; platinum analogs such as cisplatin and carboplatin; vinblastine; etoposide (VP- 16); ifosfamide; mitoxantrone; vincristine; NAVELBINE® (vinorelbine); novantrone;
teniposide; edatrexate; daunomycin; aminopterin; capecitabine (XELODA®); ibandronate; CPT-11 ; topoisomerase inhibitor RFS 2000; difluoromethylomithine (DMFO); retinoids such as retinoic acid; and pharmaceutically acceptable salts, acids and derivatives of any of the above.
[116] Also included in the definition of "chemotherapeutic agent" or "cytotoxic drug" are: (i) anti-hormonal agents that act to regulate or inhibit hormone action on tumors such as anti-estrogens and selective estrogen receptor modulators (SERMs), including, for example, tamoxifen (including NOLVADEX®; tamoxifen citrate), raloxifene, droloxifene, 4- hydroxytamoxifen, trioxifene, keoxifene, LY 117018, onapristone, and FARESTON®
(toremifine citrate); (ii) aromatase inhibitors that inhibit the enzyme aromatase, which regulates estrogen production in the adrenal glands, such as, for example, 4(5)-imidazoles, aminoglutethimide, MEGASE® (megestrol acetate), AROMASIN® (exemestane; Pfizer), formestanie, fadrozole, RIVISOR® (vorozole), FEMARA® (letrozole; Novartis), and
ARIMIDEX® (anastrozole; AstraZeneca); (iii) anti-androgens such as flutamide, nilutamide, bicalutamide, leuprolide, and goserelin; as well as troxacitabine (a 1,3-dioxolane nucleoside cytosine analog); (iv) protein kinase inhibitors; (v) lipid kinase inhibitors; (vi) antisense oligonucleotides, particularly those which inhibit expression of genes in signaling pathways implicated in aberrant cell proliferation, such as, for example, PKC-alpha, Ralf and H-Ras; (vii) ribozymes such as VEGF expression inhibitors (e.g., ANGIOZYME®) and HER2 expression inhibitors; (viii) vaccines such as gene therapy vaccines, for example,
ALLOVECTIN®, LEUVECTIN®, and VAXID®; PROLEUKIN® rIL-2; a topoisomerase 1 inhibitor such as LURTOTECAN®; ABARELIX® rmRH; (ix) anti-angiogenic agents such as bevacizumab (AVASTIN®, Genentech); and (x) pharmaceutically acceptable salts, acids and derivatives of any of the above. Other anti-angiogenic agents include MMP-2 (matrix - metalloproteinase 2) inhibitors, MMP-9 (matrix-metalloproteinase 9) inhibitors, COX-II (cyclooxygenase II) inhibitors, and VEGF receptor tyrosine kinase inhibitors. Examples of such useful matrix metalloproteinase inhibitors that can be used in combination with the present compounds/compositions are described in WO 96/33172, WO 96/27583, EP 818442, EP 1004578, WO 98/07697, WO 98/03516, WO 98/34918, WO 98/34915, WO 98/33768, WO 98/30566, EP 606,046, EP 931,788, WO 90/05719, WO 99/52910, WO 99/52889, WO 99/29667, WO 99/07675, EP 945864, U.S. Pat. No. 5,863,949, U.S. Pat. No. 5,861,510, and EP 780,386, all of which are incorporated herein in their entireties by reference.
Examples of VEGF receptor tyrosine kinase inhibitors include 4-(4-bromo-2-fluoroanilino)- 6-methoxy-7-(l-methylpiperidin-4-ylmethoxy)quinazoline (ZD6474; Example 2 within WO 01/32651), 4-(4-fluoro-2-methylindol-5-yloxy)-6-methoxy-7-(3-pyrrolidin- l-ylpropoxy)- quinazoline (AZD2171 ; Example 240 within WO 00/47212), vatalanib (PTK787; WO 98/35985) and SU11248 (sunitinib; WO 01/60814), and compounds such as those disclosed in PCT Publication Nos. WO 97/22596, WO 97/30035, WO 97/32856, and WO 98/13354).
[117] Other examples of chemotherapeutic agents / cytotoxic drugs that can be used in combination with the present compounds include inhibitors of PI3K (phosphoinositide-3 kinase), such as those reported in Yaguchi et al (2006) Jour, of the Nat. Cancer Inst.
98(8):545-556; U.S. Pat. No. 7, 173,029; U.S. Pat. No. 7,037,915; U.S. Pat. No. 6,608,056; U.S. Pat. No. 6,608,053; U.S. Pat. No. 6,838,457; U.S. Pat. No. 6,770,641 ; U.S. Pat. No.
6,653,320; U.S. Pat. No. 6,403,588; WO 2006/046031 ; WO 2006/046035; WO 2006/046040; WO 2007/042806; WO 2007/042810; WO 2004/017950; US 2004/092561 ; WO
2004/007491 ; WO 2004/006916; WO 2003/037886; US 2003/149074; WO 2003/035618; WO 2003/034997; US 2003/158212; EP 1417976; US 2004/053946; JP 2001247477; JP 08175990; JP 08176070; U.S. Pat. No. 6,703,414; and WO 97/15658, all of which are incorporated herein in their entireties by reference. Specific examples of such PI3K inhibitors include SF-1126 (PI3K inhibitor, Semafore Pharmaceuticals), BEZ-235 (PI3K inhibitor, Novartis), XL-147 (PI3K inhibitor, Exelixis, Inc.).
[118] Chemotherapeutic agents / cytotoxic drugs may also include any of the generic drugs or biosimilars of the brand-name drugs referenced herein, or improvements thereof, including improved formulations, delivery means (sustained release, bioadhesive coating, targeted delivery etc.), and dosage forms.
[119] The term "viral infection" refers to the invasion of a host organism' s bodily tissues by disease-causing viruses. Examples of the viral infections include CMV infection, HIV infection and AIDS.
[120] The term "parasite infection" refers to the invasion of a host organism's bodily tissues by disease-causing parasites. Examples of the parasite infections include giardiasis, amoebiasis, and schistosomiasis.
[121] The phrase "pharmaceutically acceptable" indicates that the substance or composition must be compatible chemically and/or toxicologically, with the other ingredients comprising a formulation, and/or the mammal being treated therewith.
[122] The term "therapeutically effective amount" means that amount of active compound or conjugate that elicits the desired biological response in a subject. Such response includes alleviation of the symptoms of the disease or disorder being treated, prevention, inhibition or a delay in the recurrence of symptom of the disease or of the disease itself, an increase in the longevity of the subject compared with the absence of the treatment, or prevention, inhibition or delay in the progression of symptom of the disease or of the disease itself. Determination of the effective amount is well within the capability of those skilled in the art, especially in light of the detailed disclosure provided herein. Toxicity and therapeutic efficacy of compound I can be determined by standard pharmaceutical procedures in cell cultures and in experimental animals. The effective amount of compound or conjugate of the present invention or other therapeutic agent to be administered to a subject will depend on the stage, category and status of the multiple myeloma and characteristics of the subject, such as general health, age, sex, body weight and drug tolerance. The effective amount of compound
or conjugate of the present invention or other therapeutic agent to be administered will also depend on administration route and dosage form. Dosage amount and interval can be adjusted individually to provide plasma levels of the active compound that are sufficient to maintain desired therapeutic effects.
CELL BINDING AGENT-DRUG MOIETY CONJUGATES
[123] In the first embodiment, the present invention provides a conjugate represented by Formula (I) below:
In addition, nineteen specific embodiments for the conjugate are further described below in this conjugate section.
[124] In a first specific embodiment, hi is represented by the following formula:
JC B' Z 1— A1— J UI '
in which:
s2 is the site covalently linked to the group U;
Ar is an optionally substituted arylene or an optionally substituted heteroarylene; Cy is an optionally substituted carbocyclic or heterocyclic ring;
R204 and R2os are each independently optionally substituted alkyl;
R301 is H or optionally substituted alkyl;
Cy' is an optionally substituted carbocyclic or heterocyclic ring;
Zi is -NR9-C(=0)- or -C(=0)-NR9- or absent,
Ai is absent, an optionally substituted alkylene, an optionally substituted
cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH2-CH2-0)pl-(CRbRc)pr- or -(CRbRC)PR-(0-CH2-CH2)pl-,
Jui' is absent, -NR9- or -C(=0)- pl is an integer from 1 to 1000 (specifically, 1 to 24, more specifically 1 to 8 (e.g, 2, 4, 6 or 8))
pi' is an integer from 0 to 10;
R9 is H or an optionally substituted alkyl; and
Ra, Rb, Rc and Re, for each occurrence, are independently H or an optionally substituted alkyl.
Alternatively, pi is 1 to 24. In another alternative, pi is 1 to 8. In yet another alternative, pi is 2, 4, 6, or 8. The remainder of the variables in Formula (I) are as described in the first embodiment.
le JCB' is
si s1 ¾—S— CRbRc-C(=0)— I s2
s 1
N
The remainder of the variables in Formula (I) or the formula designated in the first specific embodiment are as defined in the first embodiment or its first specific embodiment.
in which Ri and R2, for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10. The remainder of the variables in Formula (I) or each of the above formulas representing hi are as defined in the first embodiment or its first or second specific embodiment.
[127] In a fourth s ecific embodiment, h is represented by the following formula:
in which:
Ri and R2, for each occurrence, are each independently H or an alkyl;
m is an integer from 1 to 5; and
R9 is H or an alkyl.
The remainder of the variables in Formula (I) or each of the formulas in this specific embodiment are as defined in the first embodiment or its third specific embodiment.
[128] In a fifth specific embodiment, R and R2 are both H; and R9 is H. The remainder of the variables in Formula (I) or each of the formulas designated in the third or fourth specific embodiment are as defined in the first embodiment or its third or fourth specific embodiment. 129] In a sixth specific embodiment, hi is represented by the following formula:
. The remainder of the variables in Formula (I) or each of the formulas designated in this specific embodiment are as defined in the first embodiment or its fifth specific embodiment.
[130] In a seventh specific embodiment, L2 is represented by the following formula:
Ji_i2' A2— JQ'
in which:
JU2'is -C(=0)-, -NR9- or absent;
A2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]j.io;
JD is -C(=0)-, -C(=0)-(CRBRV-C(=0)-, -C(=0)-(CH2-CH2-0)p2-(CRb Rc')p2<-S-, -
[XX], for each occurrence, is independently an amino acid residue;
Rb and Rc , for each occurrence, are independently H or an optionally substituted alkyl (more specifically H);
Rb and Rc , for each occurrence, are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group;
p2 is an integer from 1 to 1000 (specifically 1 to 14, 1 to 8);
p2' is an integer from 1 to 10 (specifically 1 to 5); and
p2" is an integer from 1 to 10 (specifically 1 to 5).
Alternatively, Rb and Rc are H; p2 is 1-14; and p2'is 1 to 5; and in another alternative, Rb and Rc are H; p2 is 1-8; and p2'is 1 to 5. The remainder of the variables in
Formula (I) are as defined in the first embodiment or its first, second, third, fourth, fifth, or sixth specific embodiment.
[131] In an eighth specific embodiment, L2 is represented by the following formula:
O O
s5 HxXli -10— fs6 s5 !-IXXI MO-C— (CRB RC )P2.-C— s6
in which:
R12, R13, and R14, for each occurrence, are each independently H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
r is an integer from 0 to 10;
s5 is the site covalently linked to the group U; and
s6 is the site covalently linked to the group D.
The remainder of the variables in Formula (I) or each of the formulas designated in this specific embodiment are as defined in the first embodiment or its seventh specific
embodiment.
[132] In a ninth specific embodiment, the conjugate is represented by the following formula, or a pharmaceutically acceptable salt thereof:
The variables in each of the formulas designated in this specific embodiment are as defined in the first embodiment or its seventh or eighth specific embodiment.
[133] In a tenth specific embodiment, Q is i) H; ii) -S03H, -Z'-S03H, -OP03H2, -Z'- OP03H2, -P03H2, -Z'-P03H2, -C02H, -Z'-C02H, -NR10iRi02 ,-Z'-NR10iRio2, or a
Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R102, are each independently H or an optionally substituted alkyl; R103 to R105 are each independently an optionally substituted alkyl; and X" is a pharmaceutically acceptable anion. The remainder of the variables in Formula (I) or each
of the formulas designated in the eighth or ninth embodiment above are as defined in the first embodiment or its eighth or ninth specific embodiment.
[134] In an eleventh specific embodiment, R12 is H; and Q is H, SO3H or a pharmaceutically acceptable salt thereof. The remainder of the variables in Formula (I) or each of the formulas designated in the eighth or ninth embodiment above are as defined in the first embodiment or its eighth, ninth or tenth specific embodiment.
[135] In a twelfth specific embodiment, Rb and Rc are both H; and p2' is an integer from 1 to 5. The remainder of the variables in Formula (I) or each of the formulas designated in the seventh, eighth, or ninth specific embodiment are as defined in the first embodiment or its seventh, eighth, ninth, tenth, or eleventh specific embodiment.
[136] In a thirteenth specific embodiment, R13 and R14 are both H; and r is 1 to 5. The remainder of the variables in Formula (I) or each of the formulas designated in the eighth, or ninth embodiment are as defined in the first embodiment or its eighth, ninth, tenth, eleventh, or twelfth specific embodiment.
[137] In a fourteenth specific embodiment, the conjugate is represented by one of the following formulas, or a pharmaceutically acceptable salt thereof:
or
The variables in each of the formulas designated in this specific embodiment are as defined in the first embodiment.
[138] In a fifteenth specific embodiment, D is a maytansinoid in the conjugate in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth specific embodiment.
[139] In a sixteenth specific embodiment, D in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth specific embodiment is represented by:
in which:
R, R' and R", for each occurrence, are independently H or an optionally substituted alkyl; and
Y is an optionally substituted alkylene, an optionally substituted alkenylene, or an optionally substituted alkynlene.
[140] In a seventeenth specific embodiment, Y in the structural formulas designated in the sixteenth embodiment is represented by
in which:
R15 to R18, for each occurrence, are independently H or an optionally substituted alkyl, an optionally substituted alkenyl, an optionally substituted cycloalkyl, an optionally substituted heterocyclyl, an optionally substituted aryl or an optionally substituted heteroaryl; and
r is an integer between 0 to 15.
Alternatively, R15 to R^, for each occurrence, are independently H or an optionally substituted alkyl. In another alternative, R15 to R^ are all H and r is 1. In yet another alternative, R15 and R16 are H, r is 2, and R17 and R18 are both methyl. .
[141] In an eighteenth specific embodiment, D in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, fourteenth, fifteenth, sixteenth, or seventeenth specific embodiment is represented by the followin formula:
[142] In a nineteenth specific embodiment, the conjugate is represented by the following formula, or a pharmaceutically acceptable salt thereof:
50
which: DM' is represented by the following formula:
[143] In the first embodiment or each of its nineteen specific embodiments described in this section above, w is specifically an integer between 1 and 6 or alternatively, between 1 and 4.
CYTOTOXIC COMPOUND
[144] In a second embodiment, the present invention also provides a cytotoxic compound represented by Formula (II) below:
— U -(-L2-D) q >
In addition, nineteen specific embodiments for the cytotoxic compound are further described below in this cytotoxic compound section.
[145] In a first specific embodiment, hi is represented by the following formula:
JCB ~ Ai— Jui '
in which: JCB is -COX", maleimide, -SZ, X'-CRbRc-C(=0)-, X'-CRbRc-C(=0)-NRe-, Ra-C(=0)-, Ra-C(=0)-Ar-, NH2-NRe-, NH2-NRe-C(=0)-, NH2-NRe-Ar-, ΝΗ2-0-,
optionally substituted arylene or an optionally substituted heteroarylene,
is an optionally substituted cycloalkyne or an optionally substituted heterocycloalkyne, R201, R202 and R203 each are independently H or an optionally substituted alkyl; Z2 is pyridyl or
5 wherein R2o4 and R205 are each independently optionally substituted alkyl,
is an optionally substituted strained cycloalkene or an optionally substituted strained heterocycloalkene;
X' is a halogen;
X" is -OH or a carboxylic acid activating group (specifically COX" is a reactive ester);
Z is H or -SRd;
Ra, Rb, Rc and Re, for each occurrence, are independently H or an optionally substituted alkyl;
Rd is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
Zi is -NR9-C(=0)- or -C(=0)-NR9- or absent;
Ai is absent, an optionally substituted alkylene, an optionally substituted
cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH2-CH2-0)pl-(CRbRc)pl'- or -(CRbRc)pr-(0-CH2-CH2)pl-;
Jui ' is absent, -NR9- or -C(=0)-;
pi is an integer from 1 to 1000 (specifically, 1 to 24, more specifically 1 to 8 (e.g, 2, 4, 6 or 8));
pi ' is an integer from 0 to 10; and
R9 is H or an optionally substituted alkyl.
Alternatively, COX" is a reactive ester and pi is 1 to 24. In another alternative, COX" is a reactive ester and pi is 1 to 8. In yet another alternative, COX" is a reactive ester and pi is 2, 4, 6, or 8. Alternatively, The remainder of the variables in Formula (II) are as described in the second embodiment.
[146] In a second specific embodiment, variable JCB is -COX", maleimide, -SZ, X'-CRbRc- C(=0)-, X'-CRbRc-C(=0)-NRe-, Ra-C(=0)-, Ra-C(=0)-phenylene-, NH2-NR6-, NH2-NRe-
The remainder of the variables in Formula (II) or the formula designated in the first specific embodiment are as defined in the second embodiment, each of its alternative embodiments, or its first specific embodiment.
in which R and R2, for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10. The remainder of the variables in Formula (II) are as defined in the second embodiment or its first or second specific embodiment.
[148] In a fourth specific embodiment, JCB is -COX" or a maleimide; R and R2, for each occurrence, are each independently H or an alkyl; m is an integer from 1 to 5; and R9 is H or an alkyl. The remainder of the variables in Formula (II) or each of the above formulas representing are as defined in the second embodiment or its third specific embodiment.
[149] In a fifth specific embodiment, R and R2 are both H; and R9 is H. The remainder of the variables in Formula (II) or each of the above formulas representing are as defined in the second embodiment or its third or fourth specific embodiment.
[150] In a sixth specific embodiment is represented by the following formula:
The remainder of the variables in Formula (II) or each of the formulas designated in this specific embodiment are as defined in the second embodiment or its fifth specific embodiment.
[151] In a seventh specific embodiment, L2 is represented by the following formula:
Ju2' 2— JQ'
in which:
JU2'is -C(=0)-, -NR9- or absent;
A2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]1-10;
JD is -C(=0)-, -C(=0)-(CRbRV-C(=0)-, -C(=0)-(CH2-CH2-0)p2-(CRb Rc')p2<-S-,
[XX], for each occurrence, is independently an amino acid residue;
Rb and Rc , for each occurrence, are independently H or an optionally substituted alkyl (Specifically H);
Rb and Rc , for each occurrence, are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group;
p2 is an integer from 1 to 1000 (specifically 1 to 14, 1 to 8);
p2' is an integer from 1 to 10 (specifically 1 to 5); and
p2" is an integer from 1 to 10 (specifically 1 to 5).
Alternatively, Rb and Rc are H; p2 is 1-14; and p2'is 1 to 5; and in another alternative, Rb and Rc are H; p2 is 1-8; and p2'is 1 to 5. The remainder of the variables in Formula (II) are as defined in the second embodiment or its first, second, third, fourth, fifth, or sixth specific embodiment.
in which:
R12, Ri3, and R14, for each occurrence, are each independently H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
r is an integer from 0 to 10;
s5 is the site covalently linked to the group U; and
s6 is the site covalently linked to the group D.
The remainder of the variables in Formula (II) or each of the formulas designated in this specific embodiment are as defined in the second embodiment or its seventh specific embodiment.
[153] In a ninth specific embodiment, the cytotoxic compound is represented by the following formula, or a salt thereof:
The variables in each of the formulas designated in this specific embodiment are as defined in the second embodiment or its seventh or eighth specific embodiment.
[154] In a tenth specific embodiment, Q is i) H; ii) -S03H, -Z'-S03, -OP03H2, -Z'-OP03H2, -P03H2, -Z'-P03H2, -C02H, -Z'-C02H, -NR10iRio2 ,-Z'-NR10iRio2, or a pharmaceutically acceptable salt thereof; or iii)
or -Z'-N+R1o3Rio4RiosX ; Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R102, are each independently H or an optionally substituted alkyl; Rio3 to Rios are each independently an optionally substituted alkyl; and X" is a pharmaceutically acceptable anion. The remainder of the variables in Formula (II) or each of the formulas designated in the eighth or ninth embodiment above are as defined in the second embodiment or its eighth or ninth specific embodiment.
[155] In an eleventh specific embodiment, R12 is H; and Q is H, S03H or a salt (e.g., pharmaceutically acceptable salt) thereof. The remainder of the variables in Formula (II) or each of the formulas designated in the eighth or ninth embodiment above are as defined in the second embodiment or its eighth, ninth, or tenth specific embodiment.
[156] In a twelfth specific embodiment, Rb and Rc are both H; and p2' is an integer from 1 to 5. The remainder of the variables in Formula (II) or each of the formulas designated in the seventh, eighth, or ninth specific embodiment are as defined in the second embodiment or its seventh, eighth, ninth, tenth, or eleventh specific embodiment.
[157] In a thirteenth specific embodiment, R13 and R14 are both H; and r is 1 to 5. The remainder of the variables in Formula (II) or each of the formulas designated in the eighth, or ninth embodiment are as defined in the second embodiment or its eighth, ninth, tenth, eleventh, or twelfth specific embodiment.
[158] In a fourteenth specific embodiment, the cytotoxic compound is represented by one of the following formulas or a salt thereof:
The variables in each of the formulas designated in this specific embodiment are as defined in the second embodiment.
[159] In a fifteenth specific embodiment, D is a maytansinoid in the cytotoxic compound in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth specific embodiment.
[160] In a sixteenth specific embodiment, D in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth specific embodiment is represented by:
in which:
R, R' and R", for each occurrence, are independently H or an optionally substituted alkyl; and
Y is an optionally substituted alkylene, an optionally substituted alkenylene, or an optionally substituted alkynlene.
[161] In a seventeenth specific embodiment, Y in the structural formulas designated in the sixteenth embodiment is represented by
in which:
R15 to R18, for each occurrence, are independently H or an optionally substituted alkyl, an optionally substituted alkenyl, an optionally substituted cycloalkyl, an optionally substituted heterocyclyl, an optionally substituted aryl or an optionally substituted heteroaryl; and
r is an integer between 0 to 15.
Alternatively, R15 to R18, for each occurrence, are independently H or an optionally substituted alkyl. In another alternative, R15 to R18 are all H and r is 1. In yet another alternative, R15 and R16 are H, r is 2, and R17 and R18 are both methyl. .
[162] In an eighteenth specific embodiment, D in the first embodiment or its first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh, twelfth, thirteenth, fourteenth, fifteenth, sixteenth, or seventeenth specific embodiment is represented by the following formula:
[163] In a nineteenth specific embodiment, the cytotoxic compound is represented by the following formula, or a salt thereof:
LINKER COMPOUNDS
[164] In the third embodiment, the present invention further provides a linker compound represented by Formula (III) below:
Li '— U"(-L2' ) q
In addition, sixteen specific embodiments for the linker compound are further described below in this linker compound section.
[165] In a first specific embodiment, is represented by the following formula:
JCB ΑΪ Ju/
wherein:
, Ra-C(=0)-Ar-, NH2-NRe-,
, is an o
ptionally substituted arylene or an optionally substituted heteroaryle an optionally substituted cycloalkyne or an optionally substituted heterocycloalkyne, R201, R202 and R203 each are independently H or an optionally substituted alkyl; Z2 is pyridyl or
wherein R2o4 and R205 are each independently optionally substituted alkyl,
a
nd is an optionally substituted strained cycloalkene or an optionally substituted strained heterocycloalkene;
X' is a halogen;
X" is -OH or a carboxylic acid activating group (specifically COX" is a reactive ester);
Z is H or -SRd;
Ra, Rb, Rc and Re, for each occurrence, are independently H or an optionally substituted alkyl;
Rd is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
Zi is -NR9-C(=0)- or -C(=0)-NR9- or absent;
Ai is absent, an optionally substituted alkylene, an optionally substituted
cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH2-CH2-0)pl-(CRbRc)pl - or -(CRbRc)pr-(0-CH2-CH2)pl-;
Jui ' is absent, -NR9- or -C(=0)-;
pi is an integer from 1 to 1000 (specifically, 1 to 24, more specifically 1 to 8 (e.g, 2, 4, 6 or 8));
pi ' is an integer from 0 to 10; and
R9 is H or an optionally substituted alkyl.
Alternatively, COX" is a reactive ester and pi is 1 to 24. In another alternative, COX" is a reactive ester and pi is 1 to 8. In yet another alternative, COX" is a reactive ester and pi is 2,
4, 6, or 8. The remainder of the variables in Formula (III) are as described in the third embodiment.
[166] In a second specific embodiment, JCB is -COX", maleimide, -SZ, X'-CRbRc-C(=0)-, '- RbRc-C(=0)-NRe-, Ra-C(=0)-, Ra-C(=0)-phenylene-, NH2-NRe-, NH2-NRe-phenylene-,
The remainder of the variables in Formula (III) or the formula designated in the first specific embodiment are as defined in the third embodiment, each of its alternative embodiments, or its first specific embodiment.
in which, R and R2, for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10. The remainder of the variables in Formula (III) or each of the formulas described in this specific embodiment are as defined in the third embodiment or its first or second specific embodiment.
[168] In a fourth specific embodiment, JCB is -COX" or a maleimide; R and R2, for each occurrence, are each independently H or an alkyl; R9 is H or an alkyl; and m is an integer from 1 to 5. The remainder of the variables in Formula (III) or each of the formulas designated in the third specific embodiment are as defined in the third embodiment or its first, second, or third specific embodiment.
[169] In a fifth specific embodiment, R and R2 are both H; and R9 is H. The remainder of the variables in Formula (III) or each of the above formulas representing hi are as defined in the third embodiment or its third or fourth specific embodiment.
[170] In a sixth specific embodiment, h\ is represented by the following formula:
The remainder of the variables in Formula (III) or each of the formulas designated in this specific embodiment are as defined in the third embodiment or its fifth specific embodiment.
[171] In a seventh specific embodiment, L2' is represented by the following formula:
Ju2' A2— JD
wherein:
JU2'is -C(=0)-, -NR9- or absent;
A2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX] 1-10;
JD is -C(=0)-X", -C(=0)-(CRb Rc')p2-C(=0)-X", -C(=0)-(CH2-CH2-
X" is -OH or a carboxylic acid activating group;
Z is H or -SRd;
Rd is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
[XX], for each occurrence, is independently an amino acid residue;
Rb and Rc , for each occurrence, are independently H or an optionally substituted alkyl (specifically H);
Rb and Rc , for each occurrence, are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group;
p2 is an integer from 1 to 1000 (specifically 1 to 14, 1 to 8);
p2' is an integer from 1 to 10 (specifically 1 to 5);
p2" is an integer from 1 to 10 (specifically 1 to 5).
Alternatively, Rb and Rc are H; p2 is 1-14; and p2'is 1 to 5; and in another alternative, Rb and Rc are H; p2 is 1-8; and p2'is 1 to 5. The remainder of the variables in Formula (III) are
as defined in the third embodiment or its first, second, third, fourth, fifth, or sixth specific embodiment.
[172 In an eighth specific embodiment, L2' is represented by the following formula:
wherein:
R12, R13, and R14, for each occurrence, are each independently H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
r is an integer from 0 to 10; and
s5 is the site covalently linked to the group U.
The remainder of the variables in Formula (III) or each of the formulas designated in this specific embodiment are as defined in the third embodiment or its seventh specific embodiment.
[173] In a ninth specific embodiment, X" together with the adjacent -C(=0)- group is a reactive ester group; and Rd is a pyridyl or nitropyridyl. The remainder of the variables in Formula (III) or each of the formulas designated in the eighth specific embodiment are as defined in the third embodiment or its seventh or eighth specific embodiment.
[174] In a tenth specific embodiment, the compound is represented by the following formula, or a salt thereof:
The variables in each of the formulas designated in this specific embodiment are as defined in the third embodiment or its seventh, eighth, or ninth specific embodiment.
[175] In a eleventh specific embodiment, Q is i) H; ii) -SO3H, -Z'-S03H, -OP03H2, -Z'- OP03H2, -P03H2, -Z'-P03H2, -C02H, -Z'-C02H, -NRioiRio2 ,-Z'-NRioiRio2, or a
pharmaceutically acceptable salt thereof; or iii) -N+R1o3Rio4RiosX or -Z'-N^RiosRi^RiosX ; Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R102, are each independently H or an optionally substituted alkyl; Rio3 to Rios are each independently an optionally substituted alkyl; and X" is a pharmaceutically acceptable anion. The remainder of the variables in Formula (III) or each of the formulas designated in the eighth or tenth embodiment above are as defined in the third embodiment or its eighth, ninth, or tenth specific embodiment.
[176] In a twelfth specific embodiment, R12 is H; and Q is H, S03H or a salt (e.g., pharmaceutically acceptable salt) thereof. The remainder of the variables in Formula (III) or each of the formulas designated in the eighth or tenth embodiment above are as defined in the third embodiment or its eighth, ninth, tenth, eleventh specific embodiment.
[177] In a thirteenth specific embodiment, Rb and Rc are both H; and p2' is an integer from 1 to 5. The remainder of the variables in Formula (III) or each of the formulas designated in the seventh, eighth, or tenth specific embodiment are as defined in the third embodiment or its seventh, eighth, ninth, tenth, eleventh or twelfth specific embodiment.
[178] In a fourteenth specific embodiment, R13 and R14 are both H; and r is 1 to 5. The remainder of the variables in Formula (III) or each of the formulas designated in the eighth, or ninth embodiment are as defined in the third embodiment or its eighth, ninth, tenth, eleventh, twelfth, or thirteenth specific embodiment.
[179] In a fifteenth specific embodiment, wherein the compound is represented by the following formula or a salt thereof:
The variables in each of the formulas designated in this specific embodiment are as defined in the seventh specific embodiment of this section.
[180] In a sixteenth specific embodiment, in each of the formulas designated in the fifteenth specific embodiment of this section, X" together with the adjacent -(C=0)- group forms a reactive ester group; Z is -SRd, and Rd is a pyridyl or nitropyridyl.
MODIFIED CELL BINDING AGENT
[181] In a fourth embodiment, the present invention further provides a modified cell binding agent represented by Formula (IV) below:
[182] In addition, sixteen specific embodiments for the modified cell binding agent are further described below in this modified cell binding agent section.
[183] In a first specific embodiment, hi is represented by the following formula:
JC B' Z 1— A1— J UI '
in which:
s1
N
-CRbRc-C(=0)— I s2 s1 — S-CRbRc-C(=0) I-—NNRRe— I s2
s2 s1 CRz = N-NRe § s2 s1 — CHRz"NH-NRe— | s2
S1 CRZ— N— NRe— C(=0)- s2
CHRZ— N-NRe— C(=0) \ s1 " CRz- -NRe— Ar j s2 s1 -CHRZ-N- NRe— Ar s2 S1 CRz— N~0- s2
s2 is the site covalently linked to the group U;
Ar is an optionally substituted arylene or an optionally substituted heteroarylene;
Cy is an optionally substituted carbocyclic or heterocyclic ring;
R201, R202 and R203 each are independently H or an optionally substituted alkyl;
R204 and R205 are each independently optionally substituted alkyl;
R301 is H or optionally substituted alkyl;
Cy' is an optionally substituted carbocyclic or heterocyclic ring;
Zi is -NR9-C(=0)- or -C(=0)-NR9- or absent,
Ai is absent, an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH2-CH2-0)p1-(CRbRc)p1 -(CRbRc)pr-(0-CH2-CH2)pl-,
Jui ' is absent, -NR9- or -C(=0)- pl is an integer from 1 to 1000 (specifically, 1 to 24, more specifically 1 to 8 (e. 4, 6 or 8))
pi ' is an integer from 0 to 10;
R9 is H or an optionally substituted alkyl; and
R\ Rb, Rc, Re and Rz, for each occurrence, are independently H or an optionally substituted alkyl.
Alternatively, pi is 1 to 24. In another alternative, pi is 1 to 8. In yet another alternative, pi is 2, 4, 6, or 8. The remainder of the variables in Formula (IV) are as described in the fourth embodiment.
s1
phenylene— | s2 s 1 ¾ -CR2=N-NRe— I s2 31 — CHRz"NH-NRe— 5 s2
C RZ— N-NRe-phenylene— ¾ s2 s i — C HRZ— N"NRe-phenylene
H
The remainder of the variables in Formula (IV) or the formula designated in the first specific embodiment are as defined in the fourth embodiment, each of its alternative embodiments, or its first specific embodiment.
[185] In a third specific embodiment, hi is represented by the following formula:
in which R and R2, for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10. The remainder of the
variables in Formula (IV) or each of the above formulas representing Li are as defined fourth embodiment or its first or second specific embodiment.
[186] In a fourth specific embodiment, h is represented by the following formula:
in which:
Ri and R2, for each occurrence, are each independently H or an alkyl;
m is an integer from 1 to 5; and
R9 is H or an alkyl.
The remainder of the variables in Formula (IV) or each of the formulas in this specific embodiment are as defined in the fourth embodiment or its third specific embodiment.
[187] In a fifth specific embodiment, Ri and R2 are both H; and R9 is H. The remainder of the variables in Formula (IV) or each of the formulas designated in the third or fourth specific embodiment are as defined in the fourth embodiment or its third or fourth specific embodiment.
[188 In a sixth s ecific embodiment, Li is represented by the following formula:
The remainder of the variables in Formula (IV) or each of the formulas designated in this specific embodiment are as defined in the fourth embodiment or its fifth specific
embodiment.
[191] In a seventh specific embodiment, L2' is represented by the following formula:
Ju2' 2— JQ
wherein:
JU2'is -C(=0)-, -NR9- or absent;
A2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]no;
JD is -C(=0)-X", -C(=0)-(CRb Rc')p2'-C(=0)-X", -C(=0)-(CH2-CH2-
X" is -OH or a carboxylic acid activating group;
Z is H or -SRd;
Rd is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
[XX], for each occurrence, is independently an amino acid residue;
Rb and Rc , for each occurrence, are independently H or an optionally substituted alkyl (specifically H);
Rb and Rc , for each occurrence, are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group;
p2 is an integer from 1 to 1000 (specifically 1 to 14, 1 to 8);
p2' is an integer from 1 to 10 (specifically 1 to 5); and
p2" is an integer from 1 to 10 (specifically 1 to 5).
Alternatively, Rb and Rc are H; p2 is 1-14; and p2'is 1 to 5; and in another alternative, Rb and Rc are H; p2 is 1-8; and p2'is 1 to 5. The remainder of the variables in Formula (IV) are as defined in the fourth embodiment or its first, second, third, fourth, fifth, or sixth specific embodiment.
[192 In an eighth specific embodiment, L2' is represented by the following formula:
wherein:
R12, Ri3, and R14, for each occurrence, are each independently H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group; and
r is an integer from 0 to 10.
The remainder of the variables in Formula (IV) or each of the formulas designated in this specific embodiment are as defined in the fourth embodiment or its seventh specific embodiment.
[193] In a ninth specific embodiment, X" together with the adjacent -C(=0)- group is a reactive ester group; and Rd is a pyridyl or nitropyridyl. The remainder of the variables in Formula (IV) or each of the formulas designated in the eighth specific embodiment are as defined in the fourth embodiment or its seventh or eighth specific embodiment.
[194] In a tenth specific embodiment, the modified cell binding agent is represented by the following formula or a salt thereof:
The variables in each of the formulas designated in this specific embodiment are as defined in the fourth embodiment or its seventh, eighth, or ninth specific embodiment.
[195] In a eleventh specific embodiment, Q is i) H; ii) -S03H, -Z'-S03H, -OP03H2, -Z'- OP03H2, -P03H2, -Z'-P03H2, -C02H, -Z'-C02H, -NRioiRio2 ,-Z'-NRioiRio2, or a
Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R102, are each independently H or an optionally substituted alkyl; Rio3 to Rios are each independently an optionally substituted alkyl; and X" is a pharmaceutically acceptable anion. The remainder of the variables in Formula (IV) or each of the formulas designated in the eighth or tenth embodiment above are as defined in the fourth embodiment or its eighth, ninth, or tenth specific embodiment.
[196] In a twelfth specific embodiment, R12 is H; and Q is H, S03H or a salt (e.g., pharmaceutically acceptable salt) thereof. The remainder of the variables in Formula (IV) or each of the formulas designated in the eighth or tenth embodiment above are as defined in the fourth embodiment or its eighth, ninth, tenth, eleventh specific embodiment.
[197] In a thirteenth specific embodiment, Rb and Rc are both H; and p2' is an integer from 1 to 5. The remainder of the variables in Formula (IV) or each of the formulas designated in
the seventh, eighth, or tenth specific embodiment are as defined in the fourth embodiment or its seventh, eighth, ninth, tenth, eleventh or twelfth specific embodiment.
[198] In a fourteenth specific embodiment, R13 and R14 are both H; and r is 1 to 5. The remainder of the variables in Formula (IV) or each of the formulas designated in the eighth, or ninth embodiment are as defined in the fourth embodiment or its eighth, ninth, tenth, eleventh, twelfth, or thirteenth specific embodiment.
[199] In a fifteenth specific embodiment, wherein the modified cell binding agent is represented by one of the following formulas, or a salt thereof:
93
.
The variables in each of the formulas designated in this specific embodiment are as defined in the seventh specific embodiment of this section.
[200] In a sixteenth specific embodiment, in each of the formulas designated in the fifteenth specific embodiment of this section, X" together with the adjacent -(C=0)- group forms a reactive ester group; Z is -SRd, and Rd is a pyridyl or nitropyridyl.
[201] In the fourth embodiment above or each of its sixteen specific embodiments described in this section above, w is specifically an integer between 1 and 6 or alternatively, between 1 and 4.
[202] Furthermore, in the first, second, third, or fourth embodiment described above in the summary section or each of the specific embodiments described in the conjugate section, the cytotoxic compound section, the linker compound section, or the modified cell binding agent section, U in one specific embodiment is represented by the following formula:
S3
in which:
R3, R4, R5, R6, R7, Rs, Rio and Rn, for each occurrence, are independently H or an optionally substituted alkyl;
n, n' , and n" are independently an integer from 1 to 10;
V is H, an optionally substituted alkyl, N02, or -NH-C(=0)-R3U1 ;
R501 is H or an optionally substituted alkyl;
s3 is the site covalently linked the group hi; and
s4 is the site covalently linked to the group L2' .
Alternatively, R3, R4, R5, R6, R7, R%, R10 and Rn are all H. In another alternative, n, n' , and n" are independently an integer from 1 to 5. In yet another alternative, U is represented by the followin formula:
In still another alternative embodiment, V in each of the formulas designated in the above alternative embodiments is H or N02.
[203] In one embodiment, LI , LI', D, L2 and L2' do not comprise a self-immolative spacer moiety. In another embodiment, LI , LI', D, L2 and L2' do not comprise a self-immolative spacer moiety connected to a peptide moiety. In yet another embodiment, LI and LI ' do not comprise a self-immolative spacer moiety which spaces and covalently links together the drug moiety and a protein peptide moiety (in the case of LI) or the reactive functional group in LI' and a protein peptide moiety (in the case of LI'). A self-immolative spacer may be defined as a bifunctional chemical moiety which is capable of covalently linking together two spaced chemical moieties into a normally stable tripartate molecule, releasing one of said spaced chemical moieties from the tripartate molecule by means of enzymatic cleavage; and following said enzymatic cleavage, spontaneously cleaving from the remainder of the molecule to release the other of said spaced chemical moieties. In accordance with these embodiments of the present invention, there is no self-immolative spacer in LI, LI', D, L2 and L2', including no self immolative space group covalently linked at one of its ends to a protein peptide moiety to form a tripartide molecule which is stable and pharmacologically inactive in the absence of the target enzyme. Alternatively, there is no self-immolative spacer in LI covalently linked at one of its ends to a protein peptide moiety and covalently linked at its other end to the chemical reactive site of the drug moiety whose derivatization inhibits pharmacological activity, so as to space and covalently link together the protein peptide moiety and the drug moiety into a tripartate molecule which is stable and pharmacologically inactive in the absence of the target enzyme (or in the case of LI', spaces and links the protein peptide moiety and the reactive functional group in LI'). The tripartide molecule is enzymatically cleavable by such target enzyme at the bond covalently linking the spacer moiety and the protein peptide moiety. Such enzymatic cleavage, in turn, will activate the self-immolating character of the spacer moiety and initiate spontaneous cleavage of the bond covalently linking the spacer moiety to rest of the molecule, e.g., the drug moiety, to thereby effect release of the drug in pharmacologically active form.
[204] Exemplary self-immolative groups which are excluded in these embodiments are represented by Formulas (A)-(I):
Formula (A)
in which T is O, N or S,
-HN-Ri-COT Formula (B)
in which T is O, N or S, and
Rl is C1-C5 alkyl;
— N COOR2
H Formula (C)
(J. Med. Chem., 27: 1447 (1984)
in which T is O, N or S, and
Formula (D)
in which T is O, N or S,
[205]
in which T is O, S or N.
[206] As used herein "C1-C5 alkyl" is meant to include a branched or unbranched hydrocarbon chain having, unless otherwise noted, one to five carbon atoms, including but not limited to methyl, ethyl, isopropyl, n-propyl, sec-butyl, isobutyl, n-butyl and the like. Additional Examples are shown below
[207] Alternatively to the exclusion of the self-immolative group from LI , LI', D, L2 and L2, or in addition to the exclusion of the self-immolative group from LI , LI', D, L2 and L2, these groups do not comprise the peptides gly-phe-leu-gly and ala-leu-ala-leu.
PRODUCTION OF CELL-BINDING AGENT-DRUG CONJUGATES
[208] The conjugates of the present invention can be prepared according to any known method in the art. See, for example, WO 2009/134977, U.S. Patent Nos. 7,811,572,
6,441,163, 7,368,565, 8,163,888, U.S Publication Nos. 2006/0182750, 2011/0003969, 2012/0253021 and Widdison, W. C. et ah, "Semisynthetic maytansine analogues for the targeted treatment of cancer," /. Med. Chem. (2006) 49(14):4392-4408. In one embodiment, the conjugates of the present invention can be prepared by reacting a cell-binding agent with a cytotoxic compound of the invention {e.g., cytotoxic compounds of Formula (II)) having a reactive moiety capable of forming a covalent bond with the cell-binding agent to form a cell- binding agent-cytotoxic agent conjugate. The conjugate can optionally be purified. The cytotoxic compound of the invention can be generated in situ and used to react with the antibody without purification. Alternatively, the cytotoxic compound of the invention can be generated and purified before conjugating to the cell-binding agent.
[209] In another embodiment, the conjugates of the present invention can be prepared by: a) reacting a cell-binding agent with a reactive functional group attached to one of the spacers linked to a branched scaffold to form a modified cell-binding agent {e.g., that of formula (IV)); b) optionally purifying the modified cell-binding agent; c) conjugating a cytotoxic drug / chemotherapeutic agent to the modified cell-binding agent to form the cell-binding agent- cytotoxic compound conjugate of the present invention; and d) purifying the cell-binding agent-cytotoxic compound conjugate.
[210] In another embodiment, the conjugate of the present invention can be prepared by mixing together a cell-binding agent, a cytotoxic drug / chemotherapeutic agent, and a linker compound (e.g., that of formula (III)). For example, the cell-binding agent may be contacted with a cytotoxic drug / chemotherapeutic agent first to form a mixture comprising the cell-
binding agent and the cytotoxic drug / chemotherapeutic agent, followed by contacting the mixture with a linker compound (e.g., linker compounds of Formula (III)) to form the cell- binding agent-cytotoxic compound conjugate. The conjugate can then be purified.
[211] Any purification methods known in the art can be used to purify the conjugates of the present invention (see, for example, Bioconjugate Techniques, 2nd Edition by Greg T.
Hermanson, published by Academic Press, Inc., 2008). In one embodiment, the conjugates of the present invention can be purified using tangential flow filtration (TFF), non-adsorptive chromatography, adsorptive chromatography, adsorptive filtration, selective precipitation, high performance liquid chromatography (HPLC), dialysis or any other suitable purification process, as well as combinations thereof.
[212] Any suitable TFF systems may be utilized for purification, including a Pellicon type system (Millipore, Billerica, MA), a Sartocon Cassette system (Sartorius AG, Edgewood, NY), and a Centrasette type system (Pall Corp., East Hills, NY).
[213] Any suitable adsorptive chromatography resin may be utilized for purification.
Preferred adsorptive chromatography resins include hydroxyapatite chromatography, hydrophobic charge induction chromatography (HCIC), hydrophobic interaction
chromatography (HIC), ion exchange chromatography, mixed mode ion exchange
chromatography, immobilized metal affinity chromatography (IMAC), dye ligand
chromatography, affinity chromatography, reversed phase chromatography, and combinations thereof. Examples of suitable hydroxyapatite resins include ceramic hydroxyapatite (CHT Type I and Type II, Bio-Rad Laboratories, Hercules, CA), HA Ultrogel hydroxyapatite (Pall Corp., East Hills, NY), and ceramic fluoroapatite (CFT Type I and Type II, Bio-Rad
Laboratories, Hercules, CA). An example of a suitable HCIC resin is MEP Hypercel resin (Pall Corp., East Hills, NY). Examples of suitable HIC resins include Butyl-Sepharose, Hexyl-Sepaharose, Phenyl-Sepharose, and Octyl Sepharose resins (all from GE Healthcare, Piscataway, NJ), as well as Macro-prep Methyl and Macro-Prep t-Butyl resins (Biorad Laboratories, Hercules, CA). Examples of suitable ion exchange resins include SP- Sepharose, CM-Sepharose, and Q-Sepharose resins (all from GE Healthcare, Piscataway, NJ), and Unosphere S resin (Bio-Rad Laboratories, Hercules, CA). Examples of suitable mixed mode ion exchangers include Bakerbond ABx resin (JT Baker, Phillipsburg NJ).
Examples of suitable IMAC resins include Chelating Sepharose resin (GE Healthcare, Piscataway, NJ) and Profinity IMAC resin (Bio-Rad Laboratories, Hercules, CA). Examples of suitable dye ligand resins include Blue Sepharose resin (GE Healthcare, Piscataway, NJ) and Affi-gel Blue resin (Bio-Rad Laboratories, Hercules, CA). Examples of suitable affinity
resins include Protein A Sepharose resin (e.g., MabSelect, GE Healthcare, Piscataway, NJ), His-Tag metal affinity resins, anti-FLAG affinity resins, and lectin affinity resins, e.g. Lentil Lectin Sepharose resin (GE Healthcare, Piscataway, NJ), where the antibody bears appropriate lectin binding sites. Examples of suitable reversed phase resins include C4, C8, and C18 resins (Grace Vydac, Hesperia, CA).
[214] Any suitable non-adsorptive chromatography resin may be utilized for purification. For example, size-exclusion chromatography can be used for purifying the conjugates of the invention. Examples of suitable non-adsorptive chromatography resins include, but are not limited to, SEPHADEX™ G-25, G-50, G-100, SEPHACRYL™ resins (e.g. , S-200 and S- 300), SUPERDEX™ resins (e.g., SUPERDEX™ 75 and SUPERDEX™ 200), BIO-GEL® resins (e.g., P-6, P-10, P-30, P-60, and P- 100), and others known to those of ordinary skill in the art.
[215] In one embodiment, when the cell-binding agent is an epitope-tagged Avibody, the conjugate can be purified using hydroxyl apatite chromatography, size-exclusion
chromatography, tangential flow filtration, gel electrophoresis, dialysis, and affinity chromatography, specifically affinity chromatography, more specifically His-tag metal affinity chromatography and anti-FLAG M2 affinity chromatography (see, for example, US 2008/0152586 and US 2012/0171115).
[216] In another embodiment, when the cell-binding agent is a Centyrin, the conjugate can be purified using protein A purification, ammonium sulfate or ethanol precipitation, acid extraction, anion or cation exchange chromatography, phosphocellulose chromatography, hydrophobic interaction chromatography, size-exclusion chromatography, tangential flow filtration, affinity chromatography, hydroxylapatite chromatography and lectin
chromatography. Alternatively, the conjugate can be purified using HPLC. Specifically, the conjugate can be purified by using affinity chromatography, more specifically His-tag metal affinity chromatography. See, for example, US 2010/0255056, US 2010/0216708 and US 2011/0274623.
[217] In another embodiment, when the cell-binding agent is a DARPin, the conjugate can be purified by affinity chromatography, size exclusion chromatography, hydroxylapatite chromatography, tangential flow filtration, specifically affinity chromatography, more specifically His-Tag affinity chromatography. See, for example, U.S. Patent Publication Nos. 2004/0132028, 2009/0082274, 2011/0118146, and 2011/0224100, WO 02/20565 and WO 06/083275.
[218] The number of cytotoxic compound molecule bound per cell-binding agent (e.g. , antibody) molecule can be determined spectroscopically by measuring the ratio of the absorbance at 280 nm and 252 nm. An average of about 0.5 - about 20 cytotoxic
compounds/antibody molecule(s) can be linked by the methods described herein. In one embodiment, the average number of linked cytotoxic compound per cell-binding agent in the conjugate (i.e. , average w value) is about 0.5 to about 10, about 0.5 to 2 (e.g., 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, or 2.1), about 2 to about 8 (e.g., 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 3.0, 3.1, 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9,
4.0, 4.1, 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9, 5.0, 5.1, 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9, 6.0,
6.1, 6.2, 6.3, 6.4, 6.5, 6.6, 6.7, 6.8, 6.9, 7.0, 7.1, 7.2, 7.3, 7.4, 7.5, 7.6, 7.7, 7.8, 7.9, 8.0, or 8.1), about 2.5 to about 7, about 3 to about 5, about 2.5 to about 5.0 (e.g. , about 2.5, about 2.6, about 2.7, about 2.8, about 2.9, about 3.0, about 3.1, about 3.3, about 3.4, about 3.5, about 3.6, about 3.7, about 3.8, about 3.9, about 4.0, about 4.1, about 4.2, about 4.3, about 4.4, about 4.5, about 4.6, about 4.7, about 4.8, about 4.9, about 5.0), about 2.5 to about 4.0, about 3.0 to about 4.0, about 3.2 to about 4.2, or about 4.5 to 5.5 (e.g. , about 4.5, about 4.6, about 4.7, about 4.8, about 4.9, about 5.0, about 5.1, about 5.2, about 5.3, about 5.4, or about 5.5).
IN VITRO EVALUATION OF CYTOTOXICITY
[219] The cytotoxic compounds and cell-binding agent-drug conjugates of the invention can be evaluated for their ability to suppress proliferation of various cancer cell lines in vitro. The in vitro cytotoxicity assays can be conducted using methods known in the art (e.g. , Widdison, W. C. et al., "Semisynthetic maytansine analogues for the targeted treatment of cancer," J. Med. Chem. (2006) 49(14):4392-408). For example, cells to be evaluated can be exposed to the compounds or conjugates for 1-5 days and the surviving fractions of cells measured in direct assays by known methods. IC50 values can then be calculated from the results of the assays.
COMPOSITIONS AND METHODS OF USE
[220] The present invention includes a composition (e.g. , a pharmaceutical composition) comprising conjugates (e.g., conjugates of Formula (I), or cytotoxic compounds (e.g. , cytotoxic compounds of Formula (II)) described herein, and a carrier (a pharmaceutically acceptable carrier). The present invention also includes a composition (e.g., a pharmaceutical composition) comprising the conjugate of Formula (I), or the cytotoxic compound of Formula
(II), and a carrier (a pharmaceutically acceptable carrier), and further comprising a second therapeutic agent. The present compositions are useful for inhibiting abnormal cell growth or treating a proliferative disorder in a mammal (e.g. , human). The present compositions are also useful for treating an autoimmune disorder, a destructive bone disorder, a graft versus host disease, a transplant rejection, an immune deficiency, an inflammatory disease, an infectious disease, a viral disease, a fibrotic disease, a neurodegenerative disorder, a pancreatitis or kidney disease in a mammal (e.g., human).
[221] The present invention includes a method of inhibiting abnormal cell growth or treating a proliferative disorder in a mammal (e.g. , human) comprising administering to said mammal a therapeutically effective amount of conjugates (e.g., conjugates of formula (I)) or cytotoxic compounds (e.g., compounds of formula (II)) described herein, or a composition thereof, alone or in combination with a second therapeutic agent. In one embodiment, the proliferative disorder is cancer in general; alternatively, the proliferative disorder is cancer selected from the group consisting of breast cancer, colon cancer, brain cancer, prostate cancer, kidney cancer, pancreatic cancer, ovarian cancer, head and neck cancer, melanoma, colorectal cancer, gastric cancer, squamous cancer, small-cell lung cancer, nonsmall-cell lung cancer, testicular cancer, Merkel cell carcinoma, glioblastoma, neuroblastoma, a cancer of a lymphatic organ, and a hematological malignancy.
[222] Similarly, the present invention provides a method for inducing cell death in selected cell populations comprising contacting target cells or tissue containing target cells with an effective amount of the conjugates of the present invention. The target cells are cells to which the cell-binding agent of the conjugates can bind.
[223] If desired, other active agents, such as other anti-tumor agents, may be administered along with the conjugate.
[224] Cancer therapies and their dosages, routes of administration and recommended usage are known in the art and have been described in such literature as the Physician's Desk Reference (PDR). The PDR discloses dosages of the agents that have been used in treatment of various cancers. The dosing regimen and dosages of these aforementioned
chemotherapeutic drugs that are therapeutically effective will depend on the particular cancer being treated, the extent of the disease and other factors familiar to the physician of skill in the art and can be determined by the physician. The contents of the PDR are expressly incorporated herein in its entirety by reference. One of skill in the art can review the PDR, using one or more of the following parameters, to determine dosing regimen and dosages of the chemotherapeutic agents and conjugates that can be used in accordance with the teachings
of this invention. These parameters include: Comprehensive index; Manufacturer; Products (by company's or trademarked drug name); Category index; Generic/chemical index (non- trademark common drug names); Color images of medications; Product information, consistent with FDA labeling; Chemical information; Function/action; Indications &
Contraindications; Trial research, side effects, warnings.
[225] The present invention also provides methods of treating a non-cancerous condition comprising administering to a subject in need of treatment an effective amount of any of the conjugates described above. Examples of the conditions include, but not limited to, an autoimmune disorder (e.g., systemic lupus, rheumatoid arthritis, and multiple sclerosis), a graft versus host disease, a transplant rejection (e.g., a renal transplant rejection, a liver transplant rejection, a lung transplant rejection, a cardiac transplant rejection, and a bone marrow transplant rejection), an immune deficiency, an inflammatory diseases (i.e., myositis and pancreatitis), a destructive bone disorder, an infectious disease (e.g., viral infections and parasite infections), a viral disease, a fibrotic disease, a neurodegenerative disorder, or a kidney disease. In one embodiment, the condition selected from the group consisting of cancer, rheumatoid arthritis, multiple sclerosis, graft versus host disease, transplant rejection, lupus, myositis, infectious disease, and immune deficiency.
[226] Examples of in vitro uses include treatments of autologous bone marrow prior to their transplant into the same patient in order to kill diseased or malignant cells: treatments of bone marrow prior to their transplantation in order to kill competent T cells and prevent graft- versus-host-disease (GVHD); treatments of cell cultures in order to kill all cells except for desired variants that do not express the target antigen; or to kill variants that express undesired antigen.
[227] The conditions of non-clinical in vitro use are readily determined by one of ordinary skill in the art.
[228] Examples of clinical ex vivo use are to remove tumor cells or lymphoid cells from bone marrow prior to autologous transplantation in cancer treatment or in treatment of autoimmune disease, or to remove T cells and other lymphoid cells from autologous or allogenic bone marrow or tissue prior to transplant in order to prevent GVHD. Treatment can be carried out as follows. Bone marrow is harvested from the patient or other individual and then incubated in medium containing serum to which is added the cytotoxic agent of the invention, concentrations range from about 10 μΜ to 1 pM, for about 30 minutes to about 48 hours at about 37°C. The exact conditions of concentration and time of incubation, i.e., the dose, are readily determined by one of ordinary skill in the art. After incubation the bone
marrow cells are washed with medium containing serum and returned to the patient intravenously according to known methods. In circumstances where the patient receives other treatment such as a course of ablative chemotherapy or total-body irradiation between the time of harvest of the marrow and reinfusion of the treated cells, the treated marrow cells are stored frozen in liquid nitrogen using standard medical equipment.
[229] For clinical in vivo use, the cytotoxic compounds or conjugates of the invention will be supplied as a solution or a lyophilized powder that are tested for sterility and for endotoxin levels. Examples of suitable protocols of conjugate administration are as follows.
Conjugates are given weekly for 4 weeks as an intravenous bolus each week. Bolus doses are given in 50 to 1000 ml of normal saline to which 5 to 10 ml of human serum albumin can be added. Dosages will be 10 μg to 2000 mg per administration, intravenously (range of 100 ng to 20 mg/kg per day). After four weeks of treatment, the patient can continue to receive treatment on a weekly basis. Specific clinical protocols with regard to route of
administration, excipients, diluents, dosages, times, etc., can be determined by one of ordinary skill in the art as the clinical situation warrants.
[230] Suitable pharmaceutically acceptable carriers, diluents, and excipients are well known and can be determined by those of ordinary skill in the art as the clinical situation warrants. Examples of suitable carriers, diluents and/or excipients include: (1) Dulbecco's phosphate buffered saline, pH about 7.4, containing or not containing about 1 mg/ml to 25 mg/ml human serum albumin, (2) 0.9% saline (0.9% w/v NaCl), and (3) 5% (w/v) dextrose; and may also contain an antioxidant such as tryptamine and a stabilizing agent such as Tween 20.
[231] The method for inducing cell death in selected cell populations can be practiced in vitro, in vivo, or ex vivo.
ANALOGUES AND DERIVATIVES
[232] One skilled in the art of cytotoxic agents will readily understand that each of the cytotoxic agents described herein can be modified in such a manner that the resulting compound still retains the specificity and/or activity of the starting compound. The skilled artisan will also understand that many of these compounds can be used in place of the cytotoxic agents described herein. Thus, the cytotoxic agents of the present invention include analogues and derivatives of the compounds described herein.
All references cited herein and in the examples that follow are expressly incorporated by reference in their entireties.
Example 1. Synthesis of di-SPDP-Lys- -Ala-NHS for Production of Amine-Branched DMx piperidine 20% in DMF SPDP FMoc-Lys(FMoc)-OH, HBTU DIEA
MeOH
Synthesis of di-SPDP-Lys-beta-Ala-OH:
[233] Wang resin (FMoc-beta-Alanine-resin, 5 g, 3.68 mmol protected amine) was added to a solid-phase peptide reaction vessel. The material was swelled with dimethylformamide (100 mL) overnight then drained under vacuum. The resin was treated with 20% piperidine in DMF (100 ml, 3.68 mmol), agitated with nitrogen bubbling for 40 min, then drained and washed with dimethylformamide (4X lOOmL). Resin was agitated during each wash then drain thoroughly. FMoc-Lys(FMoc)-OH (2.171 g, 3.68 mmol) and O-Benzotriazole- Ν,Ν,Ν',Ν'-tetramethyl-uronium-hexafluoro-phosphate (HBTU, 3.48 g, 9.19 mmol) were dissolved in dimethylformamide (80 mL). This solution was transferred to the reaction vessel containing the resin, and treated with diisopropyl ethylamine (DIPEA, 3.20 ml, 18.4 mmol). The suspension was agitated for 2h then resin was washed with dimethyl formamide (4X lOOmL) followed by washing with dichloromethane (2X 100 mL). Resin was dried then treated with piperidine 20% in dimethylformamide (100 ml, 3.68 mmol) and allowed to
agitate for 40 min then drained and washed with dimethylformamide (4X lOOmL) while agitating resin. The resin was drained then SPDP (1.15 g, 3.68 mmol) and 1- hydroxybenzotriazol (HOBT, 3.5 g, 9.20 mmol) were dissolved in dimethylformamide (50 mL) and added to the reaction vessel followed by diisopropyl ethylamine (3.2 mL, 18.40 mmol) and agitated at room temperature for 3 hrs. in the dark. Resin was washed with dimethylformamide (4X 50mL) followed by washing with dichloromethane (2X 50 mL). The resin was then washed with methanol (100 mL). The reaction vessel was attached to a vacuum flask which was placed under vacuum overnight in the dark. A solution of 95:5 trifluoroacetic acid:deionized water (100 ml) was added to the dry resin with agitation for 4hrs. The reaction was vacuum filtered and filtrate was collected and the filter cask was washed with 95:5 trifluoroacetic acid:deionized water (2x 100 mL) under vacuum. The filtrates were combined and solvent was evaporated under vacuum. Residue was taken up in a minimum volume of dimethylformamide then purified by on an intelliflash system using a 45- g CI 8 cartridge flow rate 50 mL/min eluting with deionized water with the following gradient starting at 5% acetonitrile for 5 min and a linear ramp to 95% acetonitrile over the next 25 min. desired product fractions eluting between 18-20 min was frozen then lyophilized to give 289 mg, (12% yield) of desired product. MS [M+1] calcd. 611.8 found.
612.4.
Synthesis of di-SPDP-Lys-b-Ala-NHS (linker 1):
[234] Di-SPDP-Lys-b-Ala (289 mg, 0.472 mmol) was dissolved in a minimal amount of dimethylformamide and was treated with l-ethyl-3-(3-dimethylaminopropyl)carbodiimide) HC1 salt (EDC, 91 mg, 0.472 mmol) followed by N-hydroxysuccinimide (54.4 mg, 0.472 mmol). The reaction was purified by preparative reverse phase HPLC using a kromasil C18 10 micron 250 x 21.2 mm, flow rate 20 mL/min eluting with deionized water containing 0.1% formic acid with a linear gradient of 5% acetonitrile to 95% acetonitrle over 18 min. Fractions containing pure desired product (Rt 11.8 min) were immediately combined, frozen and lyophilized give 88.4 mg (26.4 % yield) of desired product. MS [M+1] calcd.709.9 found 709.4. 1H NMR (400 MHz, DMSO-J6) δ 1.16 - 1.37 (m, 4H), 1.48 (dt, J = 14.7, 6.0 Hz, 2H),
1.65 (dq, J = 13.6, 6.7, 6.0 Hz, 1H), 1.77 - 1.97 (m, 4H), 2.18 (t, J = 7.2 Hz, 2H), 2.24 - 2.36 (m, 2H), 2.71 - 2.91 (m, 11H), 2.98 (q, J = 6.2 Hz, 3H), 3.17 - 3.48 (m, 3H), 3.92 - 4.10 (m, 1H), 4.22 (q, J = 6.0 Hz, 1H), 7.14 - 7.28 (m, 2H), 7.71 - 7.88 (m, 5H), 8.21 (d, J = 6.5 Hz, 1H), 8.32 (d, 1H), 8.39 - 8.51 (m, 2H).
Example 2. Production of Disulfide Branched DM4
[235] A lOOmL round bottom flask was equipped with a magnetic stir bar and charged with 2,2'-dithiodipyridine (2.141 g, 9.72 mmol), ethanol (5.56 ml) and acetic acid (0.371 ml, 6.48 mmol). The brown suspension was allowed to stir for 10 min under argon at room
temperature. A solution of 2-aminoethanethiol HCl salt (0.736 g, 6.48 mmol) in ethanol (1.85 ml) was added drop wise over ~2 min. After 3.5 h solvent was evaporated under vacuum to give an oil that was purified by on an intelliflash system using a 40 g rediflash silica cartridge eluting at 40 mL/min with dichloromethane and a linear gradient of 0% -100% ethyl acetate over 20 min, remaining at 100 % ethyl acetate for 5 min then eluting with ethyl acetate with a linear gradient of 0% - 100 % methanol over 5 min. The fraction containing desired compound (Rt 27 min) was concentrated under vacuum to give 985 mg (82 % yield) of a white oily solid. HRMS [M+H] calcd. 187.0358 found 187.0356. 1H NMR (400MHz, CDC13) 2.32(t, J = 6.2 Hz, 2H), 2.47 (d, J=6.4 Hz, 2H), 4.04(s, 2H), 6.50 (ddd, J=7.4, 5.0, 1.0 Hz, 1H), 6.84(dt, J= 8.1, 0.9 Hz, 1H), 7.03-6.94(m, 1H), 7.75-7.68(m, 1H)
Synthesis of Fmoc-Glu(amidoethyl-2-(pyridin-2-yldisulfanyl))-(amidoethyl-2-(pyridin-2- yldisulfanyl)):
[236] Fmoc-Glu-OH (317 mg, 0.859 mmol) was dissolved in a minimal volume of dimethylformamide and treated with l-ethyl-3-(3-dimethylaminopropyl)carbodiimide) HC1 salt (EDC, 823 mg, 4.29 mmol) and l-Hydroxy-7-azabenzotriazole (HOAt, 580 mg, 4.29 mmol) followed by a solution of 2-(pyridin-2-yldisulfanyl)ethanamine (400mg, 2.147 mmol) in a minimal volume of dimethylformamide. After 3h the reaction was purified on an
Intelliflash system using a 24 g rediflash silica cartridge at a flow rate of 35 mL/min, eluting with dichloromethane for 2 min then with a linear gradient of 0- 100% ethyl acetate over the next 13 min followed by elution with ethyl acetate with a linear gradient of 0% methanol to 100% methanol over the next 8 min. The product containing fraction (Rt 22 min) was concentrated under vacuum to give 269 (89 % yield) of desired product. HRMS [M+H] calcd.
706.1645 found 706.1643. -Glu(amido-ethyl-2-sulfanyl DM4)-amidoethyl-s-sulfanyl-DM4):
[237] Fmoc-Glu(amidoethyl-2-(pyridin-2-yldisulfanyl))-(amidoethyl-2-(pyridin-2- yldisulfanyl)) (48.4 mg, 0.069 mmol) was dissolved in minimal volume of
dimethylformamide and treated with a solution DM4 ( mg, 0.137) in dimethylacetamide (9.4 ml). After 30 min the mixture was purified by reverse phase chromatography on an
Intelliflash system equipped with a 450 g C18 eluted at 50 mL/min with 9:5 deionized water containing 0.1% formic acid: acetonitrile for 5 min then with deionized water containing 0.1% formic acid using a linear gradient of between 5% and 95% acetonitrile over the next 25 min then at 95% acetonitrile for the next 15 min. The fraction containing pure desired
product (Rt 35 min) was frozen and lyophilized to give 96.2mg (68 % yield) of desired product. MS [M+Na] calcd. 2065.7 found 2066.9. -Glu(amido-ethyl-2-sulfanyl DM4)-amidoethyl-s-sulfanyl-DM4):
[238] FMoc-Glu(amido-ethyl-2-sulfanyl DM4)-amidoethyl-s-sulfanyl-DM4 (89.6mg, 0.044 mmol) was treated with 20% morpholine in dimethylformamide (2 mL) and allowed to stir under argon at room temperature. After 2 h the material was purified by HPLC using a 250 x 21.2 mm C8, 10 micron, Kromasil column eluting at a flow rate of 20 mL/min with deionized water containing 0.1% formic acid with a gradient of between 0%-95% acetonitrile over 18 min. The fraction containing pure product (Rt 8.8 min) was frozen and lyophilized to give 65 mg (81 % yield). HRMS [M+l] calcd. 1820.7115 found 1820.7101. -Glu(amido-ethyl-2-sulfanyl DM4)-amidoethyl-s-sulfanyl-DM4) (linker 2-(DM4)2):
[239] H-Glu(amido-ethyl-2-sulfanyl DM4)-amidoethyl-s-sulfanyl-DM4) (65mg, 0.036 mmol) was dissolved in a minimal volume of dimethylformamide and treated with BMPS (13.39 mg, 0.054 mmol) followed by diisopropyl ethylamine (9.35 μΐ, 0.054 mmol). After 2 h the material was purified by HPLC using a 250 x 21.2 10 micron, Kromasil column, eluting at 20 mL/min with deionized water using a gradient of between 5% - 95% acetonitrile over 18 min. The fraction containing desired product (Rt 17.7 min) was immediately frozen and lyophilized to yield disulfide branched maytansinoid 13.15mg (18.7 % yield) as a white solid. HRMS [M+l] calcd 986.3729 found 986.3721. 1H NMR (400 MHz, DMSO-J6) δ 0.78 (s, 6H), 1.06 - 1.20 (m, 18H), 1.25 (d, J = 13.7 Hz, 3H), 2.72 - 2.75 (m, 1H), 1.37 - 1.52 (m, 4H), 3.93 - 3.95 (m, 1H), 1.59 (s, 4H), 1.62 - 1.78 (m, 4H), 1.78 - 1.96 (m, 4H), 1.98 - 2.11 (m, 4H), 2.13 - 2.29 (m, 3H), 2.30 - 2.35 (m, 1H), 2.40 (t, J = 7.5 Hz, 3H), 2.54 - 2.68 (m, 5H), 2.72 (s, 4H), 2.80 (d, J = 9.6 Hz, 2H), 3.10 (s, 5H), 3.15 - 3.22 (m, 4H), 3.25 (s, 5H),
3.43 (s, 4H), 3.59 (t, J= 7.4 Hz, 2H), 3.92 (s, 5H), 4.00-4.15 (m, 3H), 4.53 (dd, J= 12.1, 2.8 Hz, 2H), 5.32 (q, J= 6.7 Hz, 3H), 5.49 - 5.68 (m, 3H), 5.91 (s, 2H), 6.43 - 6.55 (m, 3H), 6.56-6.69 (m, 3H), 6.87 (s, 2H), 6.99 (s, 2H),7.18 (d,J= 1.8 Hz, 2H), 7.83 - 7.96 (m, 1H), 7.96 - 8.06 (m, 1H), 8.12 (d, J = 7.9 Hz, 1H).
Example 3. Production of Peptide Branched Maytansinoid
Synthesis of BMPS-ditBu-2-aminopentanedioate:
[240] H-Glu(OtBu)-OtBu HCl salt (556 mg, 1.878 mmol) was dissolved in anhydrous dimethylformamide and treated with diisopropyl ethylamine (656 μΐ, 3.76 mmol) followed by a solution of BMPS (500 mg, 1.878 mmol) in dimethylformamide (6 mL) and allowed to proceed at room temp under argon. After 3h the material was purified using an Intelliflash system equipped with a 450 g C18 Redisep cartridge and eluting at 50 mL/min with deionized water containing 0.1% formic acid using 5% acetonitrile for 5 min then eluting with a linear gradient of between 5% - 95% acetonitrile over the next 20 min. The fraction containing desired product (Rt 24.3 min) was frozen and lyophilized to give desired product
706 mg (92 % yield) as a white solid. HRMS [M+l] calcd. 411.2126 found 411.2129. Synthesis of BMP-Glu-OH:
[241] BMPS-Glu(OtBu)-OtBu (706mg, 1.72 mmol) was treated with 95:5 TFA: deionized water (5mL). After stirring for lh under argon at room temperature solvent was removed under vacuum, residue was taken up in 1:2 toluene: acetonitrile (6 mL) vortexed then evaporated under vacuum to give desired product 500 mg, (100 % yield). HRMS [M+l] calcd. 299.0874 found 299.0876
Synthesis of May-NMA-Gly3-FMoc:
[242] Fmoc-Gly3-OH (285 mg, 0.692 mmol) was dissolved in a minimal volume of dimethylformamide and treated with l-ethyl-3-(3-dimethylaminopropyl)carbodiimide) HCl salt (EDC, 442 mg, 2.307 mmol) and a solution of MayNMA (300mg, 0.461 mmol) in dimethylformamide. MayNMA was in turn prepared as described in US 7,598,375. The reaction was allowed to proceed at room temperature under argon for 6 h. Then purified by HPLC using a 250 x 21.2 mm, C8, 10 micron Kromasil column, eluting at 20 mL/min using deionized water containing formic acid using a linear gradient of between 5% - 95% acetonitrile over 18 min. The fraction containing desired product (Rt 13.6 min) was frozen
then lyophilized to give 142 mg (29.5 % yield) of desired product as a white solid. HRMS [M+l] calcd. 1065.3983 found 1065.3956.
[243] May-NMA-Gly3-FMoc (142mg, 0.136 mmol) was treated with 20% morpholine dimethylformamide (lmL) at room temperature under argon. After 2 h the reaction was purified using an Intelliflash system equipped with a 450 g CI 8 redisep cartridge, eluting at 50 mL/min with 5% acetonitrile for 3 min then with a linear gradient of between 5% - 95% acetonitrile over the next 17 min. The fraction containing desired product (Rt 18.2 min) was frozen and lyophilized to give 55 mg (49.2 % yield) of desired product as an oil. HRMS
[M+l] calcd. 821.3483 found 821.3486.
Synthesis of BMP-Glu(Gly3-May-NMA)-Gly3-May-NMA (linker3-(MayNMA)2):
BMP-Glu-OH (2118-20) (9.08 mg, 0.030 mmol) was dissolved in anhydrous
dimethylformamide and treated with l-ethyl-3-(3-dimethylaminopropyl)carbodiimide) HC1 salt (EDC, 29.2 mg, 0.152 mmol) and l-Hydroxy-7-azabenzotriazole (HO At, 20.57 mg, 0.152 mmol) followed by MayNMA-Gly -H (50mg, 0.061 mmol). After stirring over night at room temperature the reaction was purified by HPLC using a 250 x 21.2 mm, C8, 10 micron Kromasil column, eluting at 20 mL/min using deionized water containing formic acid using a linear gradient of between 5% - 95% acetonitrile over 18 min. The fraction containing desired product (Rt 10.5 min) was frozen then lyophilized to give 11.6 mg (20 % yield) of white solid. HRMS [M+l] calcd. 1903.7482 found 1903.7493. HRMS [M+l] calcd.
1903.7482; found 1903.7493. 1H NMR (400 MHz, DMSO-d6) δ 0.76 (s, 5H), 1.12 (d, J = 6.3 Hz, 5H), 1.19 (d, J = 6.7 Hz, 5H), 1.25 (d, J = 13.7 Hz, 2H), 1.45 (dt, J = 9.9, 5.0 Hz, 3H), 1.58 (s, 4H), 1.71 (dd, J = 13.6, 7.1 Hz, 2H), 1.86 (s, 2H), 2.00 - 2.07 (m, 2H), 2.12 - 2.19
(m, 2H), 2.30 - 2.35 (m, 1H), 2.40 (t, J = 7.5 Hz, 2H), 2.52 - 2.58 (m, 1H), 2.62 - 2.74 (m, 6H), 2.77 (d, J = 9.6 Hz, 2H), 3.11 (s, 7H), 3.24 (s, 5H), 3.37 - 3.52 (m, 4H), 3.59 (t, J = 7.4 Hz, 2H), 3.66 - 3.83 (m, 7H), 3.92 (s, 5H), 3.95 - 4.01 (m, 3H), 4.01 - 4.11 (m, 3H), 4.16 (q, J = 7.2 Hz, 1H), 4.55 (dd, J = 12.0, 3.0 Hz, 2H), 5.27 (q, J = 6.8 Hz, 2H), 5.53 - 5.65 (m, 2H), 5.93 (s, 2H), 6.52 (s, 5H), 6.87 (s, 2H), 6.99 (s, 2H), 7.11 - 7.20 (m, 2H), 7.96 - 8.28 (m, 7H).
Example 4. Indirect Binding Assay
[244] Serial dilutions (triplicates) of Ab and conjugates in FACS Buffer (alpha-MEM + 2% goat serum) were made. Cells were re-suspended in FACS Buffer at the cell density of 5E+05 cells/ml). 100 μΐ^ of cells was plated in duplicate at 5E4 cells/well with a total volume of 200 μΐ^. The cells were mixed in the well of the plate and incubated on ice for 2-3 hours. The plates were spun down at 1200 rpm in the pre-chilled centrifuge for 5 mins. The supernatant was removed by quickly inverting plate. The cells were resuspended by gentle vortex in a plate with a cover and washed with 200 μΕΛνεΙΙ of cold FACS Buffer. The washing solution was removed by repeating the centrifuge step. FITC labeled secondary antibody was diluted to 1: 100 in FACS Buffer and 100 μΐ^ of the diluted antibody was added to each well on the assay plate. The plate was incubated for 1 hour on ice without exposing to light and then spun down and washed as described above. The cells were resuspended in 200 μΐ^ of Fixer (lxPBS with 1% Formaldehyde). The reading was taken on the FACS Calibur. As shown in Figures 24 and 25, the conjugates bind equally well as the naked antibody with similar Kd values.
Example 5. Cytotoxicity Assay
[245] Cells were plated at 2000 to 10,000 cells/well (depending on growth characteristics) in 96 well flat bottom tissue culture plates in lOOuL volume. Conjugates/Drugs were titrated in a reservoir then added to the cells on the plate, total well volume 200 μΐ^ (100 μΐ^ cells, 100 μΐ^ Conjugate/ drug titration). Each dilution of drug was in triplicate. Appropriate controls of un-dosed cells and medium only were included. Only the inner 60 wells were used; the outer wells were filled with buffer (HBSS or PBS to reduce evaporation). The plates were incubated at 37°C. with 5% C02 for 4 to 7 days depending on the growth characteristics of the cells tested. Develop when the untreated control reached confluence or when media started to change color and was becoming depleted. At the end of the incubation period WST-8 (Cat# CK04-20 Dojindo Molecular Technologies) was added at 20
to each assay well, and the plate was returned to the incubator for 2-7 hours depending on the dye turnover rate of the particular cells used. The plate was read on a plate reader at 450-650 nm. 450-650 reading was subtracted to correct for background and then the signal of the media alone is subtracted from all the wells. The results of the samples was subtracted by those of the untreated sample and plotted. In Figures 26-28, the conjugate containing a branched linking group (Ab-linker2-(DM4)2 or Ab-linker3-(MayNMA)2) is more potent than a conjugate with non-branched linking group, Ab-SPDB-DM4. In addition, the potency of the conjugates significantly decreases when the cells were blocked with naked antibodies, indicating target- specific cytotoxicity of the conjugates.
Example 6.
N-hydroxysuccinimide ester of Z-Lys(Z)-OH: Z-Lys(Z)-OH (2g, 4.83mmol) was dissolved in dimethylformamide (10 mL) and treated with l-hydroxypyrrolidine-2,5-dione (0.833 g, 7.24 mmol) followed by EDC (0.925 g, 4.83 mmol). The reaction was allowed to proceed at room temperature, under argon atmosphere overnight to give crude N- hydroxysuccinimide ester which was used without purification.
Z-Lys(Z)-Cysteic Acid: Cysteic Acid (2.434 g, 14.47 mmol) was weighed into a 250 mL beaker equipped with a stir bar and dissolved in a solution of deionized water (70 ml) and dimethylformamide (30 ml). The mixture was stirred while 1M NaOH was added to bring the pH to 8.4. Crude Z-Lys(Z)-NHS (2.468 g, 4.82 mmol) in dimethylformamide (1 mL) was added dropwise while keeping the pH between 7.5-8.4 with the addition of 1M NaOH. After addition the reaction was allowed to stir for lh. The crude mixture was concentrated under vacuum and purified using a Combiflash Rf 200i, PF 30 C18 HP 450g column with flow rate of 125 ml/min, eluting with deionized water containing 0.1% formic acid with a 5% to 95% linear acetonitrile gradient over 23 min. Fractions containing desired product were combined, frozen and lyophilized to give 2.09 g (77 % yield) of desired product. MS [M - H] calcd 564.17; found 564.17.
Z-Lys(Z)-Cys-P-Ala-OtBu: Η-β-Ala-OtBu (241 mg, 1.328 mmol) was dissolved in dimethylsulfoxide (10 mL), then treated with diisopropyl ethylamine (309 μΐ, 1.771 mmol) followed by Z-Lys(Z)-Cysteic (497 mg, 0.718 mmol, 81 % yield). After all solids were in solution the reaction was treated with PyBOP (461 mg, 0.886 mmol). The reaction was allowed to proceed at room temperature under an argon atmosphere for 1 h and was quenched with water. The crude material was purified using a Combiflash Rf 200i, PF 30 CI 8 HP 450g column with flow rate of 125ml/min. Eluting with deionized water containing 0.1% formic acid using a linear gradient of acetonitrile 10% to 95% over 35 min. Fractions containing desired product were combined, frozen and lyophilized to give 497mg (81% yield) of desired product as an oily solid. HRMS [M-H]" calcd. 693.3; found 693.3.
Lys-Cys-P-Ala-OH: Z-Lys(Z)-Cysteic Acid- -Ala-OtBu (497mg, 0.718 mmol) was treated with 95:5 TFA:Water (10 mL) under an argon atmosphere overnight. The material was co- evaporated with toluene (3x 5 mL). The crude product was purified purified using a
Combiflash Rf 200i, PF 30 C18 AQ 175g column with flow rate of 75ml/min. Eluting with
deionized water containing 0.1% formic acid for 5 min then using a linear gradient of acetonitrile 0% - 20% over 15 min then 20% to 95% over 10 min. Fractions containing desired material were combined, frozen then lyophilized to give 74 mg (28.0 % yield) of desired product as a white solid. HRMS [M+ H]+
SPDB-Lys(SPDB)-Cys-P-Ala: Lys-Cys- -Ala-OH (37mg, 0.101 mmol) was dissolved in dimethyl sulfoxide ( 1 mL) and treated with a solution of SPDB (72.3 mg, 0.222 mmol) dissolved in dimethyl sulfoxide (1 mL) and followed by diisopropyl ethylamine (0.035 ml, 0.201 mmol). The reaction was allowed to proceed at room temperature under an Argon atmosphere for 3h then was purified by semi-preparative C18 HPLC using a XB-C18 21.2 x 150mm, 5μιη column with a flow rate of 21.2mL/min. Eluting with deionized water containing 0.1% formic acid with an acetonitrile gradient as follows: 5% acetonitrile for 3 min then a linear gradient from 3% - 90% acetonitrile over 17 min. The desired product eluted at 6.4 minutes and was collected, frozen then lyophilized to give 39.7mg (49.9 % yield) of desired product as a white solid. HRMS [M+H]+ calcd. 791.2; found 791.2.
N-hydroxysuccinimide ester of SPDB-Lys(SPDB)-Cys- -Ala (linker 4): SPDB- Lys(SPDB)-Cys- -Ala-OH (39.7mg, 0.050 mmol) was dissolved in dimethylforaiamide (1 mL), to which was added EDC (9.63 mg, 0.050 mmol) and N-hydroxysuccinimide (5.78 mg, 0.050 mmol). The reaction was allowed to proceed at room temperature under argon atmosphere for 2 h then purified by semi-preparative CI 8 HPLC using a XB-C18
21.2x150mm, 5μηι column with a flow rate of 21.2mL/min. eluting with deionized water containing 0.1% formic acid using a gradient of acetonitrile 5% for 4 min then a linear gradient of 5% - 95% over 17 min. The desired product eluted at 5.6 minutes and was collected, immediatly frozen and lypholized to give 12.7 mg (28.5 % yield) of product as a white solid. MS [M+H]+ calcd. 888.19; found 888.18. 1H NMR (400 MHz, DMSO-J6) δ 1.16
- 1.37 (m, 4H), 1.48 (dt, J = 14.7, 6.0 Hz, 2H), 1.65 (dq, J = 13.6, 6.7, 6.0 Hz, 1H), 1.77 - 1.97 (m, 4H), 2.18 (t, J = 7.2 Hz, 2H), 2.24 - 2.36 (m, 2H), 2.71 - 2.91 (m, 11H), 2.98 (q, J = 6.2 Hz, 3H), 3.17 - 3.48 (m, 3H), 3.92 - 4.10 (m, 1H), 4.22 (q, J = 6.0 Hz, 1H), 7.14 - 7.28 (m, 2H), 7.71 - 7.88 (m, 5H), 8.21 (d, J = 6.5 Hz, 1H), 8.32 (d, 1H), 8.39 - 8.51 (m, 2H).
Z-Lys(Z)-b-Ala-OtBu: Z-L-Lys(Z)-OH (10.0 g, 24 mmol) was dissolved in dimethyl formamide (100 mL) to which was added beta-alanine t-butyl ester HCl salt (5.0 g, 27 mmol), EDC (6.0 g, 31 mmol) and HOBT (4.0 g, 25 mmol). After reacting for 2 h the reaction was purified directly on a 1200 g C18 cartridge on an intelliflash system, eluting with deionized water containing 0.2% formic acid with a gradient of 5 - 95% acetonitrile over 50 min at a flow rate of 100 mL/min. Fractions containing desired product were combined, frozen and lyophilized to give 8 g, (58 % yield) of desired product as a white solid. HRMS [MS + H]+ Calcd. 542.2861 ; found 542.2862. 1H NMR (400 MHz, Chloroform-J) δ 1.35 (t, J = 1.6 Hz, 2H), 1.44 (s, 9H), 1.50 (dt, J = 13.7, 6.7 Hz, 2H), 1.57 - 1.69 (m, 1H), 1.70 - 1.88 (m, 2H),
2.42 (t, J = 6.2 Hz, 2H), 3.12 - 3.20 (m, 2H), 3.46 (q, J = 6.1 Hz, 2H), 4.09 (q, J = 7.2 Hz, 1H), 4.88 (s, 1H), 5.08 (d, J = 6.7 Hz, 4H), 5.51 (d, J = 7.8 Hz, 1H), 6.58 (d, J = 7.2 Hz, 1H), 7.24 - 7.39 (m, 10H).
H-Lys-b-Ala-OtBu: Z-Lys(Z)-b-Ala-OtBu (7.7 g, 14.2 mmol) was dissolved in 1 :2 methanol :dimethoxyethane (150 mL) containing deionized water (8 mL) in a 250 mL capacity PAR shaker flask, to which was added 10% Palladium on carbon (500 mg). The flask was shaken under 40 PSI hydrogen pressure for 3 h then the mixture was vacuum filtered through celite filter aid. Filtrate solvent was removed under vacuum to give 3.8 g (96% ield) of desired product as a thick oil. HRMS [MS + H]+ 274.2125; found 274.2122. 1H NMR (400 MHz, Acetonitrile-d3) δ 1.21 (d, J = 6.9 Hz, 7H), 1.38 (s, 9H), 1.49 - 1.73 (m, 2H), 2.38 (t, J = 6.8 Hz, 2H), 2.76 (d, J = 0.8 Hz, 2H), 2.90 (s, 2H), 3.10 (t, J = 6.8 Hz, 2H), 3.30 (dt, J = 9.4, 6.8 Hz, 2H), 3.48 (qd, J = 7.0, 1.3 Hz, 2H), 3.76 (s, 1H), 3.82 - 3.86 (m, 5H), 4.15 (dd, J = 9.3, 4.9 Hz, 1H).
(Z-Ala-Gly-Gly)2-Lys-b-Ala-OtBu: H-Lys-b-Ala-OtBu (4.65 g, 17 mmol) and Z-Ala-Gly-
Gly-OH (14.34 g, 42 mmol) were dissolved in anhydrous dimethyl formamide (60 mL) to which was added EDC (9.78 g, 51 mmol) and the mixture was magnetically strirred for 3 h. approximately 5 mL of deionized water was added to solubilize material then the sample was vacuum filtered and loaded onto a 450 g CI 8 cartridge using the Intelliflash system. The cartridge was pre-equilibrated at 90% deionized water containing 0.2% formic acid and 10% acetonitrile. The cartridge was eluted with deionized water containing 0.2% formic acid and a linear gradient of 10 - 95% acetonitrile over 40 min. Fractions containing desired product were combined, frozen and lyophilized to give 8 g (51% yield) of a white solid. HRMS
[M+Na]+ calcd. 934.4281 ; found 934.4260. 1H NMR (400 MHz, Acetonitrile-J3) δ 1.39 (s, 3H), 1.41 (s, 3H), 1.49 (s, 9H), 1.73 (dddd, J = 44.5, 17.6, 8.5, 4.8 Hz, 2H), 2.47 (t, J = 6.9 Hz, 2H), 3.20 (q, J = 6.1 Hz, 2H), 3.29 - 3.48 (m, 2H), 3.59 (s, 6H), 3.79 - 3.97 (m, 8H), 4.14 - 4.32 (m, 3H), 5.02 - 5.24 (m, 4H), 6.87 (s, 1H), 7.34 - 7.51(m, 10H), 7.52 - 7.69 (m, 1H).
(H-Ala-Gly-Gly)2-Lys-b-Ala-OtBu: (Z-Ala-Gly-Gly)2-Lys-b-Ala-OtBu (11 g, 12 mmol) was vigorously stirred with 9: 1 methanol: water (250 mL) and dimethylformamide (50 mL). Some material did not go into solution. 10%Pd-C (430 mg) was then added and the sample was hydrogenated in a 500 mL Par hydrogenator flask at 40 PSI of hydrogen for 3 h. The mixture was vacuum filtered through celite filter aid. Deionized water 60 ml was added to the filtrate and the combined solution was frozen then lyophilized. About 2 h into the lyophilization the material thawed but was left on the lyophilizer for 3 days giving 5 g (64% yield) of white solid. HRMS [M + H]+ calcd. 644.3740; found 644.3726.
(H-Ala-Gly-Gly)-Lys-OH: (H-Ala-Gly-Gly)2-Lys-OtBu (2.8 g, 4.3 mmol) was taken up in
9: 1 TFA:deionized water (20 mL) and magnetically stirred for 2 h. Solvent was evaporated under vacuum and the residue was taken up in deionized 2: 1 acetonitrile: deionized water (50 mL), frozen and lyophilized to give 2.6 grams (95% yield) of an oily solid as the TFA salt.
HRMS [M + H]+ calcd. 588.3101 ; found 588.3100. 1H NMR (400 MHz, Deuterium Oxide) δ
1.29 (ddt, J = 16.1, 9.3, 6.0 Hz, 1H), 1.47 (q, J = 7.3 Hz, 1H), 1.52 (d, J = 7.1 Hz, 3H), 1.70
(dddd, J = 26.3, 17.6, 9.1, 5.3 Hz, 1H), 2.57 (t, J = 6.5 Hz, 1H), 2.82 (d, J = 0.8 Hz, OH), 2.98
(s, OH), 3.16 (t, J = 6.9 Hz, 1H), 3.43 (dtd, J = 18.4, 13.6, 6.5 Hz, 1H), 3.90 (d, J = 27.5 Hz, 2H), 3.97 - 4.07 (m, 2H), 4.09 - 4.23 (m, 2H).
Example 8.
(DMl-GMB-Ala-Gly2)-Lys-b-Ala-OH: DM1 (101 mg, 140 mmol) and H-Ala-Gly-Gly)2- Lys-b-Ala-OH (40 mg, 0.068 mmol) were dissolved in anhydrous dimethyl formamide (1.0 mL) to which was added deionized water (10 μί). The mixture was magnetically stirred vigorously GMBS (39 mg, 0.14 mmol) in anhydrous dimethyl formamide was added followed by diisopropyl ethyl amine (50 μί, 0.28 mmol) and the reaction was stirred for 1 hour then purified on a 27 mm x 220 mm CI 8 load and lock HPLC column flow rate 40 mL/min eluting with deionized water containing 0.2% formic acid and a linear gradient of 5 - 95% acetonitrile over 30 min. Fractions containing pure desired product were combined and frozen then lypohilized to give 40 mg (24% yield) of desired product as white solid. MS [M + Na]+ calcd. 2414.9; found 2415.2. 1H NMR (400 MHz, DMSO-J6) δ 0.78 (d, J = 2.1 Hz, 6H), 1.12 (d, J = 6.3 Hz, 6H), 1.18 (t, J = 6.5 Hz, 12H), 1.25 (d, J = 12.8 Hz, 3H), 1.36 (t, J = 7.6 Hz, 2H), 1.45 (tt, J = 10.0, 5.5 Hz, 5H), 1.59 (s, 6H), 1.64 (dd, J = 12.1, 5.9 Hz, 4H), 2.00 - 2.14 (m, 6H), 2.29 - 2.38 (m, 3H), 2.71 (s, 5H), 2.79 (dd, J = 9.7, 1.8 Hz, 3H), 2.85 (d, J = 6.2 Hz, 2H), 2.86 - 2.96 (m, 4H), 2.96 -3.06 (m, 4H), 3.10 (s, 6H), 3.14 - 3.22 (m, 4H), 3.25 (s, 8H), 3.49 (d, J = 9.0 Hz, 4H), 3.65 (d, J = 5.8 Hz, 2H), 3.72 (dd, J = 12.1, 5.7 Hz, 6H), 3.85 (dd, J = 9.0, 3.9 Hz, 1H), 3.88 - 3.97 (m, 7H), 4.01 - 4.19 (m, 3H), 4.19 - 4.28 (m, 2H), 4.52 (dd, J = 12.1, 2.9 Hz, 2H), 5.26 - 5.36 (m, 2H), 5.56 (td, J = 10.0, 8.9, 3.3 Hz, 2H), 5.92 (s, 2H), 6.49 - 6.61 (m, 6H), 6.88 (s, 2H), 7.17 (dd, J = 8.5, 1.8 Hz, 2H), 7.70 (t, J = 5.6 Hz, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.95 (t, J = 5.5 Hz, 1H), 7.99 - 8.14 (m, 4H), 8.21 (dt, J = 17.2, 6.0 Hz, 2H).
(DMl-GMB-Ala-Gly-Gly)-Lys-b-Ala-Mal: (DM 1 -GMB- Ala-Gly-Gly)2-Lys-b-Ala-OH (5.3 mg, 0.0022 mmol) was dissolved in anhydrous dimethylformamide (0.25 mL) to which was added EDC (2.0 mg, 0.014 mmol). After 2 min 2-amino-ethyl-maleimide HC1 salt (1 mg, 0.0057 mmol) was added and the solution was stirred at room temperature for 15 min. The reaction mixture was purified by HPLC on a XB-C18 21.2x150 mm, 5μιη column with a flow rate of 21.2mL/min. eluting with deionized water containing 0.1% formic acid using a gradient of acetonitrile 5% for 4 min then a linear gradient of 5% - 95% over 17 min.
Fractions containing desired product were combined, frozen and lyophilized to give 2 mg (35 % yield) of white solid. MS [M + Na]+ calcd. 2537.0; found 2537.3.
(SPDB-A-GG)2-Lys-b-Ala-OH: (H-Ala-Gly-Gly)2-Lys-b-Ala-OH (450 mg, 0.76 mmol) was dissolved in anhydrous dimethyl formamide (11 mL) to which was added SPDB (683 mg, 2.1 mmol) and diisopropyl amine (450 μί, 3 mmol). After 2h the reaction was injected directly onto a 19 mm x 150 mm C18 HPLC column eluting with deionized water containing 0.2% formic acid with a 5 - 95% acetonitrile linear gradient with a flow rate of 20 mL/min.
Fractions containing desired product were combined, frozen and lyophilized to give 230 mg, (30% yield) of desired product as a whitre solid. HRMS [M + Na]+ calcd. 1032.3171; found 1032.3194. 1H NMR (400 MHz, DMSO-J6) δ 1.19 (d, J = 7.1 Hz, 3H), 1.23 (d, J = 8.1 Hz, 1H), 1.36 (h, J = 6.7, 5.7 Hz, 1H), 1.41 - 1.55 (m, 1H), 1.60 (dt, J = 13.4, 7.5 Hz, 1H), 1.84 (p, J = 1.4 Hz, 2H), 2.25 (td, J = 7.3, 2.6 Hz, 2H), 2.35 (t, J = 1.0 Hz, 1H), 2.83 (t, J = 1.3 Hz, 2H), 3.01 (q, J = 6.6 Hz, 1H), 3.22 (dh, J = 19.9, 6.6 Hz, 2H), 3.61 - 3.76 (m, 4H), 4.19 (dtd,
J = 32.4, 8.5, 7.8, 5.2 Hz, 2H), 7.23 (dd, J = 7.3, 4.8 Hz, 1H), 7.73 - 7.98 (m, 3H), 8.02 (t, J = 5.8 Hz, 1H), 8.09 - 8.25 (m, 2H), 8.42 - 8.48 (m, 1H).
(SPDB-Ala-Gly-Gly)2-Lys-b-Ala-ONHS: (SPDB-Ala-Gly-Gly)2-Lys-b-Ala-OH (200 mg, 0.19 mmol) N-hydroxysuccinimide (68 mg, 0.59 mmol) and EDC (114 mg, 0.59 mmol) were dissolved in anhydrous dimethyl formamide (2 mL) and magnetically stirred at ambient temperature for 2 hours, and the reaction mixture was puified by HPLC on a 27 cm x 220 cm CI 8 column flow rate 40 mL/min eluting with deionized water containing 0.1% formic acid using a 5 - 95% acetonitrile linear gradient over 28 min. Fractions containing pure desired product were combined, frozen and lyophilized to give 82 mg (39% yield) of desired product as a white solid. HRMS [M + Na]+ calcd. 1129.3334; found 1129.3325. 1H NMR (400 MHz, DMSO-J6) 5 1.19 (d, J = 7.1 Hz, 6H), 1.26 (d, J = 9.3 Hz, 1H), 1.36 (p, J = 7.0, 6.5 Hz, 2H), 1.49 (td, J = 9.0, 4.7 Hz, 1H), 1.63 (dt, J = 13.2, 6.6 Hz, 1H), 1.85 (p, J = 7.4 Hz, 6H), 2.26 (td, J = 7.3, 2.1 Hz, 4H), 2.32 (t, J = 7.3 Hz, 2H), 2.72 (t, J = 7.2 Hz, 2H), 2.77 - 2.89 (m, 9H), 3.01 (q, J = 6.7 Hz, 2H), 3.34 (ddq, J = 26.2, 13.0, 6.5 Hz, 3H), 3.65 (d, J = 5.8 Hz, 2H), 3.70 (d, J = 5.1 Hz, 3H), 3.74 (d, J = 5.1 Hz, 2H), 4.20 (dtd, J = 28.4, 8.5, 7.9, 5.1 Hz, 3H), 7.19 - 7.27 (m, 2H), 7.68 (t, J = 5.6 Hz, 1H), 7.76 (d, J = 8.0 Hz, 2H), 7.79 - 7.86 (m, 2H), 7.91 (d, J = 8.0 Hz, 1H), 8.01 (q, J = 6.2 Hz, 2H), 8.11 (dt, J = 12.4, 6.3 Hz, 3H), 8.18 (t, J = 5.8 Hz, 2H), 8.45 (dd, J = 4.9, 1.7 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 17.73, 17.84, 22.70, 24.02, 24.48, 25.43, 28.72, 30.34, 31.57, 32.17, 33.43, 34.25, 36.92, 37.49, 37.51, 38.41, 41.98, 42.09, 42.19, 48.32, 48.40, 52.48, 119.14, 121.07, 137.73, 149.51, 159.27, 167.24, 168.30, 168.51, 169.00, 169.17, 170.07, 171.39, 171.48, 171.76, 172.83, 172.95, 173.93.
Example 10.
(SPDB-Ala-Gly-Gly)2-Lys-P-Ala-Mal: (SPDB-Ala-Gly-Gly)2-Lys-P-Ala-ONHS (22 mg, 0.020 mmol) was dissolved in anhydrous dimethyl formamide (400 μί) to which was added DM4 (30 mg, 0.038) with magnetic stirring. After 2 min deionized water (20 μί) was added then after 15 min diisopropylethyl amine (10 μί, mmol) and H2N-(CH2)2-Mal HC1 salt (3.7 mg, 0.022 mmol) were added. After an additional 30 min, sample was injected directly onto a 19 mm x 150 mm C18 HPLC column eluting with deionized water containing 0.2% formic acid with a 5-95% linear acetonitrile gradient. Flow rate was 20 mL/min. Fractions containing pure desired product were combined and frozen then lyophilized to give 19 mg (38% yield) of product as a white solid. MS [M + Na]+ calcd.2491.0; found 2491.3.
Claims
wherein:
CBA is a cell binding agent;
hi is a spacer;
U is a branched scaffold;
L2, for each occurrence, is independently a spacer
D, for each occurrence, is independently a cytotoxic drug moiety;
q is an integer from 2 to 5; and
w is an integer between 1 and 10.
2. The conjugate of claim 1, wherein hi is represented by the following formula:
JC B' Z 1— A1— J UI '
wherein:
s 1 CRz = N-NR— C(=0) S2 S1 I CHRZ_H_NRe_c(=o) s2
in which si is the site covalently linked to the CBA, s2 is the site covalently linked to the group U, Ar is an optionally substituted arylene or an optionally substituted heteroarylene, Cy is an optionally substituted carbocyclic or heterocyclic ring, R201 , R202 and R203 each are independently H or an optionally substituted alkyl; Z2 is pyridyl
or
5 wherein R2o4 and R205 are each independently optionally substituted alkyl, R301 is H or optionally substituted alkyl; and Cy' is an optionally substituted carbocyclic or heterocyclic ring;
Zi is -NR9-C(=0)- or -C(=0)-NR9- or absent,
Ai is absent, an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH2-CH2- 0)pi-(CRbRc)pr- or -(CRbRc)pr-(0-CH2-CH2)pl-,
Jui ' is absent, -NR9- or -C(=0)- pl is an integer from 1 to 1000
pi ' is an integer from 0 to 10;
R9 is H or an optionally substituted alkyl; and
Ra, Rb, Rc,Re, and Rz for each occurrence, are independently H or an optionally substituted alkyl.
-CRbRc-C(=0)-NRe— 1 s2
s1
phenylene— S2 s1 >— CRZ— N— NRE | S2 S1 I CHRZ-NH— NRE | s2 s1 1_CRZ— N"NRe-phenylene— ¾ s2 s ς— CHRZ— N~NRe-p he ny lene— J s2
wherein R and R2, for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
The conjugate of any one of claims 1-4, wherein hi is represented by the following formula:
wherein:
Ri and R2, for each occurrence, are each independently H or an alkyl; m is an integer from 1 to 5; and
R9 is H or an alkyl.
The conjugate of claim 4 or 5, wherein Ri and R2 are both H; and R9 is H.
The conju ate of claim 6, wherein hi is represented by the following formula:
The conjugate of any one of claims 1-7, wherein L2 is represented by the following formula:
wherein:
JU2'is -C(=0)-, -NR9- or absent;
A2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]1-10;
JD' is -C(=0)-, -C(=0)-(CRbRc)p2-C(=0)-, -C(=0)-(CH2-CH2- 0)p2-(CRb'Rc')p2<- S-, -S-, absent, -C(=0)-(CRb"Rc")p2-S- or
(
C(=0) -(CRb"Rc")p2..— N'
[XX], for each occurrence, is independently an amino acid residue;
Rb and Rc , for each occurrence, are independently H or an optionally substituted alkyl ;
Rb and Rc , for each occurrence, are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group;
p2 is an integer from 1 to 1000;
p2' is an integer from 1 to 10; and
p2" is an integer from 1 to 10 (specifically 1 to 5).
The conjugate of claim 8, wherein L2 is represented by the following formula:
O O
s5 l-[XX] i -io- s6 s5 HxX] i-io-C— (CRb Rc )p2.-C— f s6 i 4) r— S— ; s6
wherein:
R12, Ri3, and R14, for each occurrence, are each independently H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
r is an integer from 0 to 10;
s5 is the site covalently linked to the group U; and
s6 is the site covalently linked to the group D.
10. The conjugate of claim 8 or 9, wherein the conjugate is represented by the following formula, or a pharmaceutically acceptable salt thereof:
■
11. The conjugate of claim 9 or 10, wherein Q is i) H; ii) -S03H, -Z'-S03, -OP03H2, -Z'- OP03H2, -P03H2, -Z'-P03H2, -C02H, -Z'-C02H, -NRioiRio2 ,-Z'-NRioiRio2, or a pharmaceutically acceptable salt thereof; or iii) -N+R1o3Rio4Rios or
-Z'-N^RiosRiwRiosX Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; R10i and R102, are each independently H or an optionally substituted alkyl; Rio3 to Rios are each independently an optionally substituted alkyl; and X" is a pharmaceutically acceptable anion.
12. The conjugate of any one of claims 9-11, wherein R12 is H; and Q is H, S03H or a pharmaceutically acceptable salt thereof.
13. The conjugate of any one of claims 8 -12, wherein Rb and Rc are both H; and p2' is an integer from 1 to 5.
14. The conjugate of any one of claim 9-13, wherein R1 and R14 are both H; and r is 1 to 5.
15. The conjugate of any one of claims 8-14, wherein [XX]1_1o is [XX]2_5.
16. The conjugate of any one of claims 8-15, wherein [XX], for each occurrence, is the residue of an amino acid independently selected from the group consisting of:
histidine, alanine, isoleucine, arginine, leucine, asparagine, lysine, aspartic acid, methionine, cysteine, phenylalanine, glutamic acid, threonine, glutamine, tryptophan, glycine, valine, proline, serine, tyrosine, N-methyl-histidine, N-methyl-alanine, N- methyl-isoleucine, N-methyl-arginine, N-methyl-leucine, N-methyl-asparagine, N- methyl-lysine, N-methyl-aspartic acid, N-methyl-methionine, N-methyl-cysteine, N- methyl-phenylalanine, N-methyl-glutamic acid, N-methyl-threonine, N-methyl- glutamine, N-methyl-tryptophan, N-methyl-glycine, N-methyl-valine, N-methyl- proline, N-methyl-serine, N-methyl-tyrosine, hydroxyproline, γ-carboxyglutamate, selinocysteine, O-phosphoserine, homoserine, norleucine, methionine sulfoxide,
methionine methyl sulfonium, citrulline, ornithine, cysteine sulfonic acid, cysteine sulfinic acid, 3-aminoalanine, 3-dimethylaminoalanine, 2-amino-4- (dimethylamino)butanoic acid, 2,4-diaminobutanoic acid, 2-amino-6- (dimethylamino)hexanoic acid, 2-amino-5-(dimethylamino)pentanoic acid, and β- alanine, each independently as an L or D isomer.
The conjugate of any one of claims 8-16, wherein [XX]1-10 is selected from the group consisting of: Val-Cit, Val-Lys, Phe-Lys, Lys-Lys, Ala-Lys, Phe-Cit, Leu-Cit, Lle- Cit, Trp, Cit, Phe-Ala, Phe-N9-tosyl-Arg, Phe-N9-nitro-Arg, Phe-Phe-Lys, D-Phe-Phe- Lys, Gly-Phe-Lys, Leu- Ala-Leu, He- Ala-Leu, Val-Ala-Val, Ala-Leu- Ala-Leu, β- Ala- Leu-Ala-Leu, Gly-Phe-Leu-Gly, Val-Arg, Arg-Val, Arg-Arg, Val-D-Cit, Val-D-Lys, Val-D-Arg, D- Val-Cit, D-Val-Lys, D- Val-Arg, D-Val-D-Cit, D-Val-D-Lys, D- Val-D- Arg, D-Arg-D-Arg, Ala- Ala, Ala-D-Ala, D- Ala- Ala, and D-Ala-D-Ala, Gly-Gly-Gly, Ala- Ala- Ala, D- Ala-Ala-Ala, Ala-D-Ala- Ala, Ala- Ala-D-Ala, Ala- Val-Cit, and Ala- Val-Ala.
18. The conjugate of any one of claims 8-16, wherein [XX]1-10 is Gly-Gly-Gly, Gly-Gly- Ala, Val-Ala, Glu-Ala, or Glu(OMe)-Ala.
19. The conjugate of claims 1-18, wherein the CBA is bound to hi by a residue of a thiol group from a cysteine on the CBA, a residue of a amino group from a lysine on the CBA, or a residue of a carboxylic group from glutamic acid or Aspartic acid on the
20. The conjugate of claim 19, wherein the CBA is bounded to hi by a residue of a thiol group from a cysteine on the CBA.
21. The conjugate of claim 1, wherein the conjugate is represented by one of the
following formulas, or a pharmaceutically acceptable salt thereof:
141
142
ı43
The conjugate of any one of claims 1-21, wherein D is a maytansinoid.
The conjugate of any one of claims 1-21, wherein D is represented by:
wherein:
R, R' and R", for each occurrence, are independently H or an optionally substituted alkyl; and
Y is an optionally substituted alkylene, an optionally substituted alkenylene, or an optionally substituted alkynlene.
24. The conjugate of claim 23, wherein Y is represented by
wherein R15 to R18, for each occurrence, are independently H or an optionally substituted alkyl, an optionally substituted alkenyl, an optionally substituted cycloalkyl, an optionally substituted heterocyclyl, an optionally substituted aryl or an optionally substituted heteroaryl; and r is an integer between 0 to 15.
25. The conjugate of claim 24, wherein R15 to R18, for each occurrence, are independently H or an optionally substituted alkyl.
26. The conjugate of claim 25, wherein R15 to R18 are all H and r is 1.
27. The conjugate of claim 25, wherein R15 and R16 are H, r is 2, and R17 and R18 are both methyl.
29. The conjugate of claim 1, wherein the conjugate is represented by the following formula, or a pharmaceutically acceptable salt thereof:
147
148
149
wherein DM' is represented by the following formula:
30. The conjugate of any one of claims 1-29, wherein the cell-binding agent is an
antibody, a single chain antibody, an antibody fragment that specifically binds to the target cell, a monoclonal antibody, a single chain monoclonal antibody, a monoclonal antibody fragment that specifically binds to a target cell, a chimeric antibody, a chimeric antibody fragment that specifically binds to the target cell, a domain antibody, a domain antibody fragment that specifically binds to the target cell, a lymphokine, a hormone, a vitamin, a growth factor, a colony stimulating factor, or a nutrient-transport molecule.
31. The conjugate of claim 30, wherein the cell-binding agent is a monoclonal antibody, a single chain monoclonal antibody, or a monoclonal antibody fragment that specifically binds to a target cell.
The conjugate of 30 or 31, wherein the antibody is a resurfaced antibody, a resurfaced single chain antibody, or a resurfaced antibody fragment.
The conjugate of any one of claims 30, wherein the antibody is a humanized antibody, a humanized single chain antibody, or a humanized antibody fragment.
The conjugate of any one of claims 1-29, wherein the cell-binding agent is a minibody, a diabody, a tribody, a tetrabody, a nanobody, a probody, a domain body, a unibody, a bispecific antibody, an ankyrin repeat protein (e.g. , a DARPin), a Centyrin, or an Avibody.
The conjugate of any one of claims 1-9, 11-20, 22-28, and 30-34, wherein U is represented by the following formula:
S3
wherein:
R3, R4, R5, R6, R7, R8, Rio and Rn, for each occurrence, are independently H or an optionally substituted alkyl;
n, n', and n" are independently an integer from 1 to 10;
V is H, an optionally substituted alkyl, N02, or -NH-C(=0)-R501;
R501 is H or an optionally substituted alkyl;
R12 is H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
s3 is the site covalently linked the group hi ; and
s4 is the site covalently linked to the group L2.
36. The conjugate of claim 35, wherein R3, R4, R5, R6, R7, R%, R10 and Rn are all H.
The conjugate of claim 35 or 36, wherein n, n', and n" are independently an integ from 1 to 5.
The conjugate of claim 35, wherein U is represented by the following formula:
The conjugate of any one of claims 35-38, wherein V is H or N02.
A cytotoxic compound represented by the following formula, or a salt thereof:
Li '— L -D) q wherein:
hi is a spacer attached to a reactive functional group that can form a covalent bond with a cell-binding agent;
U is a branched scaffold;
L2, for each occurrence, is independently a spacer
D, for each occurrence, is independently a cytotoxic drug moiety;
q is an integer from 2 to 5.
The cytotoxic compound of claim 40, wherein is represented by the following formula:
JcB ~ Ai— Jui '
wherein:
JCB is -COX", maleimide, -SZ, X'-CRbRc-C(=0)-, X'-CRbRc-C(=0)-NRe-, R C(=0)-, Ra-C(=0)-Ar-, NH2-NRe-, NH2-NRe-C(=0)-, NH2-NRe-Ar-, ΝΗ2-0-,
, wh is an optionally substituted arylene or an optionally substituted h
eteroarylene, an optionally substituted cycloalkyne or an optionally substituted heterocycloalkyne, R201, R202 and R2o3 each are independently H or an
0 optionally substituted alkyl; Z2 is pyridyl or ^aosO -ί· ; wherein R2o4 and R2os
are each independently optionally substituted alkyl, and
is an optionally substituted strained cycloalkene or an optionally substituted strained
heterocyclo alkene ;
X' is a halogen;
X" is -OH or a carboxylic acid activating group;
Z is H or -SRd;
Ra, Rb, Rc and Re, for each occurrence, are independently H or an optionally substituted alkyl;
RRdd iiss alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
Zi is -NR9-C(=0)- or -C(=0)-NR9- or absent;
Ai is absent, an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH2-CH2- 0)pi-(CRbRc)pr- or -(CRbRc)pr-(0-CH2-CH2)pi-;
½' is absent, -NR9- or -C(=0)-;
pi is an integer from 1 to 1000;
pi' is an integer from 0 to 10; and
R9 is H or an optionally substituted alkyl.
42. The cytotoxic compound of claim 41, wherein JCB is -COX", maleimide, -SZ, X'- CRbRc-C(=0)-, X'-CRbRc-C(=0)-NRe-, Ra-C(=0)-, Ra-C(= -phenylene-, NH2-NRe-,
The cytotoxic compound of any one of claims 40-42, wherein Li' is represented by the following formula:
wherein Ri and R2, for each occurrence, is H, an optionally substituted alkyl, charged substituent or an ionizable group; and m is an integer from 1 to 10.
The cytotoxic compound of claim 43, wherein:
JCB is -COX" or a maleimide;
Ri and R2, for each occurrence, are each independently H or an alkyl;
m is an integer from 1 to 5; and
R9 is H or an alkyl.
The cytotoxic compound of claim 43 or 44, wherein R and R2 are both H; and R9 H.
The cytotoxic compound of claim 45, wherein is represented by the following formula:
The cytotoxic compound of any one of claims 40-46, wherein L2 is represented by the following formula:
Ji_i2' A2— JQ'
wherein:
JU2'is -C(=0)-, -NR9- or absent;
A2 is an optionally substituted alkylene, an optionally substituted
cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]1-10;
JD' is -C(=0)-, -C(=0)-(CRb Rc')p2'-C(=0)-, -C(=0)-(CH2-CH2- -(CRb'Rc')p2'-S-, -S-, absent, -C(=0)-(CRb"Rc")p2-S- or
[XX], for each occurrence, is independently an amino acid residue;
Rb and Rc , for each occurrence, are independently H or an optionally substituted alkyl;
Rb and Rc , for each occurrence, are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group;
p2 is an integer from 1 to 1000;
p2' is an integer from 1 to 10;
p2" is an integer from 1 to 10 (specifically 1 to 5).
wherein:
R12, Rn, and R14, for each occurrence, are each independently H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
r is an integer from 0 to 10;
s5 is the site covalently linked to the group U; and
s6 is the site covalently linked to the group D.
49. The cytotoxic compound of claim 47 or 48, wherein the cytotoxic compound is
represented by the following formula, or a salt thereof:
50. The cytotoxic compound of claim 48 or 49, wherein Q is i) H; ii) -SO3H, -Z'-S03, - OP03H2, -Z'-OP03H2, -P03H2, -Z'-P03H2, -C02H, -Z'-C02H, -NRi0iRi02 ,-Z'- NR1oiRio2, or a pharmaceutically acceptable salt thereof; or iii) -N+R1o3Rio4RiosX or -Z'-N^RiosRiwRios Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R102, are each independently H or an optionally substituted alkyl; R103 to R105 are each independently an optionally substituted alkyl; and X" is a pharmaceutically acceptable anion.
51. The cytotoxic compound of any one of claims 48-50, wherein R12 is H; and Q is H, S03H or a pharmaceutically acceptable salt thereof.
52. The cytotoxic compound of any one of claims 47-51, wherein Rb and Rc are both H; and p2' is an integer from 1 to 5.
53. The cytotoxic compound of any one of claim 47-52, wherein R13 and R14 are both H; and r is 1 to 5.
54. The cytotoxic compound of any one of claims 47-53, wherein [XX]1-10 is [XX]2_5.
55. The cytotoxic compound of any one of claims 47-54, wherein [XX], for each
occurrence, is the residue of an amino acid independently selected from the group consisting of: histidine, alanine, isoleucine, arginine, leucine, asparagine, lysine, aspartic acid, methionine, cysteine, phenylalanine, glutamic acid, threonine, glutamine, tryptophan, glycine, valine, proline, serine, tyrosine, N-methyl-histidine,
N-methyl-alanine, N-methyl-isoleucine, N-methyl-arginine, N-methyl-leucine, N- methyl-asparagine, N-methyl-lysine, N-methyl-aspartic acid, N-methyl-methionine,
N-methyl-cysteine, N-methyl-phenylalanine, N-methyl-glutamic acid, N-methyl- threonine, N-methyl-glutamine, N-methyl-tryptophan, N-methyl-glycine, N-methyl- valine, N-methyl-proline, N-methyl- serine, N-methyl-tyrosine, hydroxyproline, γ- carboxyglutamate, selinocysteine, O-phosphoserine, homoserine, norleucine, methionine sulfoxide, methionine methyl sulfonium, citrulline, ornithine, cysteine
sulfonic acid, cysteine sulfinic acid, 3-aminoalanine, 3-dimethylaminoalanine, 2- amino-4-(dimethylamino)butanoic acid, 2,4-diaminobutanoic acid, 2-amino-6- (dimethylamino)hexanoic acid, 2-amino-5-(dimethylamino)pentanoic acid, and β- alanine, each independently as an L or D isomer.
The cytotoxic compound of any one of claims 47-54, wherein [XX]1-10 is selected from the group consisting of: Val-Cit, Val-Lys, Phe-Lys, Lys-Lys, Ala-Lys, Phe-Cit, Leu-Cit, Lle-Cit, Trp, Cit, Phe-Ala, Phe-N9-tosyl-Arg, Phe-N9-nitro-Arg, Phe-Phe- Lys, D-Phe-Phe-Lys, Gly-Phe-Lys, Leu- Ala-Leu, Ile-Ala-Leu, Val-Ala-Val, Ala-Leu- Ala-Leu, β-Ala-Leu-Ala-Leu, Gly-Phe-Leu-Gly, Val-Arg, Arg-Val, Arg-Arg, Val-D- Cit, Val-D-Lys, Val-D-Arg, D-Val-Cit, D-Val-Lys, D- Val-Arg, D-Val-D-Cit, D-Val- D-Lys, D- Val-D-Arg, D-Arg-D-Arg, Ala- Ala, Ala-D-Ala, D-Ala-Ala, and D-Ala-D- Ala, Gly-Gly-Gly, Ala- Ala- Ala, D- Ala-Ala-Ala, Ala-D-Ala-Ala, Ala-Ala-D-Ala, Ala- Val-Cit, and Ala-Val-Ala.
The cytotoxic compound of claim 47-54, wherein [XX]1-10 is Gly-Gly-Gly, Gly-Gly- Ala, Val-Ala, Glu-Ala, or Glu(OMe)-Ala.
The cytotoxic compound of claim 40, wherein the cytotoxic compound is represented by one of the followin formulas, or a salt thereof:
59. The cytotoxic compound of any one of claims 40-58, wherein D is a maytansinoid.
60. The c totoxic compound of any one of claims 40-59, wherein D is represented by:
wherein:
R, R' and R", for each occurrence, are independently H or an optionally substituted alkyl; and
Y is an optionally substituted alkylene, an optionally substituted alkenylene, or an optionally substituted alkynlene.
61. The cytotoxic compound of claim 60, wherein Y is represented by - (CR15R16)rCRnR18-, wherein R15 to R18, for each occurrence, are independently H or an optionally substituted alkyl, an optionally substituted alkenyl, an optionally substituted cycloalkyl, an optionally substituted heterocyclyl, an optionally substituted aryl or an optionally substituted heteroaryl; and r is an integer between 0 to 15.
62. The cytotoxic compound of claim 61, wherein R15 to R18, for each occurrence, are independently H or an optionally substituted alkyl.
63. The cytotoxic compound of claim 62, wherein R15 to R18 are all H and r is 1.
64. The cytotoxic compound of claim 62, wherein R15 and R16 are , r is 2, and R17 and R18 are both methyl.
65. The cytotoxic compound of any one of claims 40-60, wherein D is represented by the following formula:
The cytotoxic compound of claim 40, wherein the cytotoxic compound is represented by the followin formula, or a salt thereof:
wherein: DM' is represented b the following formula:
67. The cytotoxic compound of any one of claims 40-48, 50-57, and 59-65, wherein U is represented by the following formula:
S3
R3, R4, R5, R6, R7, R8, Rio and Rn, for each occurrence, are independently H or an optionally substituted alkyl;
n, n', and n" are independently an integer from 1 to 10;
V is H, an optionally substituted alkyl, N02, or -NH-C(=0)-R501;
R is H or an optionally substituted alkyl;
R12 is H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
s3 is the site covalently linked the group hi; and
s4 is the site covalently linked to the group L2.
The cytotoxic compound of claim 67, wherein R3, R4, R5, R6, R7, R%, R10 and Rn are all H.
The cytotoxic compound of claim 67 or 68, wherein n, n' and n" are independently an integer from 1 to 5.
The cytotoxic compound of claim 67, wherein U is represented by the following formula:
The cytotoxic compound of any one of claims 67-70, wherein V is H or N02.
A linker compound represented by the following formula, or a salt thereof:
hi is a spacer attached to a reactive functional group that can form a covalent bond with a cell binding agent;
U is a branched scaffold;
L2', for each occurrence, is independently a spacer attached to a reactive functional group that can form a covalent bond with a cytotoxic agent; and
q is an integer from 2 to 5.
The linker compound of claim 72, wherein hi is represented by the following formula:
~ Ai— Ju i '
wherein:
JCB is -COX", maleimide, -SZ, X'-CRbRc-C(=0)-, X'-CRbRc-C(=0)-NRe-, Ra- C(=0)-, Ra-C(=0)-Ar-, NH2-NRe-, NH2-NRe-C(=0)-, NH2-NRe-Ar-, ΝΗ2-0-,
, wherein Ar is an optionally substituted arylene or an optionally substituted
heteroarylene,
is an optionally substituted cycloalkyne or an optionally substituted heterocycloalkyne, R201, R202 and R203 each are independently H or an
optionally substituted alkyl; Z2 is pyridyl or erein R2o4 and R205
are each independently optionally substituted
alkyl, and is an optionally substituted strained cycloalkene or an optionally substituted strained
heterocyclo alkene ;
X' is a halogen;
X" is -OH or a carboxylic acid activating group;
Z is H or -SRd;
Ra, Rb, Rc and Re, for each occurrence, are independently H or an optionally substituted alkyl;
Rd is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
Zi is -NR9-C(=0)- or -C(=0)-NR9- or absent;
Ai is absent, an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH2-CH2- 0)pl-(CRbRc)pr- or -(CRbRC)PR-(0-CH2-CH2)pl-;
Jtn ' is absent, -NR9- or -C(=0)-;
pi is an integer from 1 to 1000 ;
pi' is an integer from 0 to 10; and
R9 is H or an optionally substituted alkyl.
The linker compound of claim 73, wherein JCB is -COX", maleimide, -SZ, X'- CRbRc-C(=0)-, X'-CRbRc-C(=0)-NRe-, Ra-C(=0)-, Ra-C( -phenylene-, NH2-NR6-,
The linker compound of any one of claims 72-74, wherein Li' is represented by the following formula:
wherein Ri and R2, for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
The linker compound of claim 75, wherein:
JCB is -COX" or a maleimide;
Ri and R2, for each occurrence, are each independently H or an alkyl;
R9 is H or an alkyl; and
m is an integer from 1 to 5.
The linker compound of claim 75 or 76, wherein Ri and R2 are both H; and R9 is H. The linker compound of claim 77, wherein Li' is represented by the following formula:
The linker compound of any one of claims 72-78, wherein L2' is represented by the following formula:
wherein:
JU2'is -C(=0)-, -NR9- or absent;
A2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]1-10;
JD is -C(=0)-X", -C(=0)-(CRb Rc')p2'-C(=0)-X", -C(=0)-(CH2-CH2 -(CRbRc)p2<-SZ,-SZ, absent, -C(=0)-(CRb"Rc")p2»-SZ or
X" is -OH or a carboxylic acid activating group;
Z is H or -SRd;
Rd is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl nitropyridyl;
[XX], for each occurrence, is independently an amino acid residue;
Rb and Rc , for each occurrence, are independently H or an optionally substituted alkyl;
Rb and Rc , for each occurrence, are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group;
p2 is an integer from 1 to 1000;
p2' is an integer from 1 to 10;
p2" is an integer from 1 to 10 (specifically 1 to 5).
The linker compound of claim 79, wherein L2' is represented by the following formula:
R12, Ri3, and Rw, for each occurrence, are each independently H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
r is an integer from 0 to 10; and
s5 is the site covalently linked to the group U.
The linker compound of claim 79 or 80, wherein X" together with the adjacent - C(=0)- group is a reactive ester group; Z is SRd; and Rd is a pyridyl or nitropyridyl. The linker compound of any one of claims 79-81, wherein the compound is represented by the followin formula, or a salt thereof:
The linker compound of any one of claims 80-82, wherein Q is i) H; ii) -SO3H, -Z'- S03, -OP03H2, -Z'-OP03H2, -P03H2, -Z'-P03H2, -C02H, -Z'-C02H, -NR10iRi02 ,-Z'- NRioiRio2, or a pharmaceutically acceptable salt thereof; or iii) -N+R1o3Rio4Rios or -Z'-N^RiosRiwRios "; Z' is an optionally substituted alkylene, an optionally
substituted cycloalkylene or an optionally substituted phenylene; R101 and R102, are each independently H or an optionally substituted alkyl; R103 to R105 are each independently an optionally substituted alkyl; and X" is a pharmaceutically acceptable anion.
84. The linker compound of any one of claims 80-83, wherein R12 is H; and Q is H, S03H or a pharmaceutically acceptable salt thereof.
85. The linker compound of any one of claims 79-84, wherein Rb and Rc are both H; and p2' is an integer from 1 to 5.
86. The linker compound of any one of claims 80-85, wherein R1 and R14 are both H; and r is 1 to 5.
87. The linker compound of any one of claims 79-86, wherein [XX] O is [XX]2_5.
88. The linker compound of any one of claims 79-87, wherein [XX], for each occurrence, is the residue of an amino acid independently selected from the group consisting of: histidine, alanine, isoleucine, arginine, leucine, asparagine, lysine, aspartic acid, methionine, cysteine, phenylalanine, glutamic acid, threonine, glutamine, tryptophan, glycine, valine, proline, serine, tyrosine, N-methyl-histidine, N-methyl-alanine, N- methyl-isoleucine, N-methyl-arginine, N-methyl-leucine, N-methyl-asparagine, N- methyl-lysine, N-methyl-aspartic acid, N-methyl-methionine, N-methyl-cysteine, N- methyl-phenylalanine, N-methyl-glutamic acid, N-methyl-threonine, N-methyl- glutamine, N-methyl-tryptophan, N-methyl-glycine, N-methyl-valine, N-methyl- proline, N-methyl-serine, N-methyl-tyrosine, hydroxyproline, γ-carboxyglutamate, selinocysteine, O-phosphoserine, homoserine, norleucine, methionine sulfoxide, methionine methyl sulfonium, citrulline, ornithine, cysteine sulfonic acid, cysteine sulfinic acid, 3-aminoalanine, 3-dimethylaminoalanine, 2-amino-4- (dimethylamino)butanoic acid, 2,4-diaminobutanoic acid, 2-amino-6- (dimethylamino)hexanoic acid, 2-amino-5-(dimethylamino)pentanoic acid, and β- alanine, each independently as an L or D isomer.
89. The linker compound of any one of claims 79-87, wherein [XX] O is selected from the group consisting of: Val-Cit, Val-Lys, Phe-Lys, Lys-Lys, Ala-Lys, Phe-Cit, Leu- Cit, Lle-Cit, Trp, Cit, Phe-Ala, Phe-N9-tosyl-Arg, Phe-N9-nitro-Arg, Phe-Phe-Lys, D- Phe-Phe-Lys, Gly-Phe-Lys, Leu-Ala-Leu, Ile-Ala-Leu, Val-Ala-Val, Ala-Leu- Ala- Leu, β-Ala-Leu-Ala-Leu, Gly-Phe-Leu-Gly, Val-Arg, Arg-Val, Arg-Arg, Val-D-Cit, Val-D-Lys, Val-D-Arg, D-Val-Cit, D- Val-Lys, D- Val-Arg, D-Val-D-Cit, D-Val-D- Lys, D-Val-D-Arg, D-Arg-D-Arg, Ala- Ala, Ala-D-Ala, D-Ala-Ala, and D-Ala-D-Ala,
Gly-Gly-Gly, Ala- Ala-Ala, D-Ala-Ala-Ala, Ala-D-Ala-Ala, Ala-Ala-D-Ala, Ala-Val- Cit, and Ala-Val- Ala.
90. The linker compound of claims 79-87, wherein [XX]i_io is Gly-Gly-Gly, Gly-Gly- Ala, Val-Ala, Glu-Ala, or Glu(OMe)-Ala.
91. The linker compound of claim 79, wherein the compound is represented by the
followin formula, or a salt thereof:
The linker compound of claim 91, wherein X" together with the adjacent -(C=0)- group forms a reactive ester group; Z is -SRd, and Rd is a pyridyl or nitropyridyl. The linker compound of any one of claims 72-81 and 83-90, wherein U is represented by the following formula:
S3
R3, R4, R5, R6, R7, Rs, Rio and Rn, for each occurrence, are independently H or an optionally substituted alkyl;
n, n', and n" are independently an integer from 1 to 10;
V is H, an optionally substituted alkyl, N02, or -NH-C(=0)-R501;
R is H or an optionally substituted alkyl;
R12 is H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
s3 is the site covalently linked the group hi ; and
s4 is the site covalently linked to the group L2.
94. The linker compound of claim 93, wherein R3, R4, R5, R6, R7, R10 and Rn are all H.
95. The linker compound of claim 93 or 94, wherein n, n', and n" are independently an integer from 1 to 5.
96. The linker com ound of claim 93, wherein U is represented by the following formula:
97. The linker compound of any one of claims 93-96, wherein V is H or N02.
wherein:
Li is a spacer;
U is a branched scaffold;
L2' , for each occurrence, is independently a spacer attached to a reactive functional group that can form a covalent bond with a cytotoxic agent;
q is an integer from 2 to 5; and
w is an integer between 1 and 10.
The modified cell binding agent of claim 98, wherein Li is represented by the following formula:
JC B' Z 1— A1— J UI '
wherein:
in which si is the site covalently linked to the CBA, s2 is the site covalently linked to the group U, Ar is an optionally substituted arylene or an optionally substituted heteroarylene, Cy is an optionally substituted carbocyclic ring or an optionally substituted heterocyclic ring, R2oi , R202 and R203 each are independently H or an
optionally substituted alkyl; Z2 is pyridyl or
? wherein R204 and R205 are each independently optionally substituted alkyl, R301 is H or optionally substituted alkyl; and Cy' is the non-alkene residue of an optionally substituted strained cycloalkene or an optionally substituted strained heterocycloalkene;
Zi is -NR9-C(=0)- or -C(=0)-NR9- or absent,
Ai is absent, an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, -(CH2-CH2- 0)pl-(CRbRc)pr- or -(CRbRC)PR-(0-CH2-CH2)pl-,
Jui ' is absent, -NR9- or -C(=0)- pl is an integer from 1 to 1000;
pi ' is an integer from 0 to 10;
R9 is H or an optionally substituted alkyl; and
Ra, Rb, Rc, Re and Rz, for each occurrence, are independently H or an optionally substituted alkyl.
si s2 s1 — S— CRbRc-C(=0)— j s2
I ¾ § |_| s
s1 I- CRZ— N-NRe-phenylene— s2 s 1 — -C HRZ— N"NRe-phenylene— 5 s2
The modified cell binding agent of any of the claims 98-100, wherein Li represented by the follo ing formula:
wherein Ri and R2, for each occurrence, is H, an optionally substituted alkyl, a charged substituent or an ionizable group; and m is an integer from 1 to 10.
102. The modified cell binding agent of any one of claims 98-101, wherein hi is
represented b the following formula:
wherein:
Ri and R2, for each occurrence, are each independently H or an alkyl;
R9 is H or an alkyl; and
m is an integer from 1 to 5.
103. The modified cell binding agent of claim 101 or 102, wherein Ri and R2 are both H; and R9 is H.
104. The modified cell binding agent of claim 103, wherein Li is represented by the followin formula:
105. The modified cell binding agent of any one of claims 98-104, wherein L2' is
represented by the following formula:
Ju2' 2— J Q
wherein:
JU2'is -C(=0)-, -NR9- or absent;
A2 is an optionally substituted alkylene, an optionally substituted cycloalkylene, an optionally substituted cycloalkylalkylene, or [XX]1-10;
JD is -C(=0)-X", -C(=0)-(CRb Rc')p2'-C(=0)-X", -C(=0)-(CH2-CH2- -(CRb'Rc' )p2' - SZ, -SZ, absent, -C(=0)-(CRb"Rc")p2-SZ or
X" is -OH or an carboxylic acid activating group;
Z is -H or -SRd;
Rd is alkyl, phenyl, nitrophenyl, dinitrophenyl, carboxynitrophenyl, pyridyl or nitropyridyl;
[XX], for each occurrence, is independently an amino acid residue; Rb and Rc , for each occurrence, are independently H or an optionally substituted alkyl;
Rb and Rc , for each occurrence, are independently H, an optionally substituted alkyl or a charged substituent or an ionizable group;
p2 is an integer from 1 to 1000 ;
p2' is an integer from 1 to 10; and
p2" is an integer from 1 to 10 (specifically 1 to 5).
6. The modified cell binding agent of claim 105, wherein L2' is represented by the
following formula:
F¾
;/ N\ /(CRi3Rl4)r SZ s5 |-[XX] i-i o- " s5 -[XX] i-i o-C— (CRb'Rc')p Ρ22.·-C— X"
Q 112 ; or
R12, Ri3, and R14, for each occurrence, are each independently H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
r is an integer from 0 to 10; and
s5 is the site covalently linked to the group U.
107. The modified cell binding agent of claim 105 or 106, wherein X" together with the adjacent -C(=0)- group is a reactive ester group; Z is SRd; and Rd is a pyridyl or nitropyridyl.
108. The modified cell binding agent of any one of claims 105-107, wherein the modified cell binding agent is represented by the following formula, or a salt thereof:
109. The modified cell binding agent of any one of claims 106-108, wherein Q is i) H; ii) - S03H, -Z' -S03H, -OP03H2, -Z' -OP03H2, -P03H2, -Z' -P03H2, -C02H, -Z' -C02H, - NR1oiRio2 -Z'-NR1oiRio2, or a pharmaceutically acceptable salt thereof; or iii) - N+R1o3Rio4Rio5 or -Z'-N^RiosRi^Rios Z' is an optionally substituted alkylene, an optionally substituted cycloalkylene or an optionally substituted phenylene; Rioi and R102, are each independently H or an optionally substituted alkyl; R103 to R105 are each independently an optionally substituted alkyl; and X" is a pharmaceutically acceptable anion.
110. The modified cell binding agent of claim 109, wherein R12 is H; and Q is H, S03H or a pharmaceutically acceptable salt thereof.
111. The modified cell binding agent of any one of claims 106 -110, wherein Rb and Rc are both H; and p2' is an integer from 1 to 5.
112. The modified cell binding agent of any one of claims 105-111, wherein R13 and R14 are both H; and r is 1 to 5.
113. The modified cell binding agent of any one of claims 105- 112, wherein [XX] 1-10 is
[XX]2-5.
114. The modified cell binding agent of any one of claims 105-113, wherein [XX] is the residue of an amino acid independently selected from the group consisting of:
histidine, alanine, isoleucine, arginine, leucine, asparagine, lysine, aspartic acid, methionine, cysteine, phenylalanine, glutamic acid, threonine, glutamine, tryptophan, glycine, valine, proline, serine, tyrosine, N-methyl-histidine, N-methyl-alanine, N- methyl-isoleucine, N-methyl-arginine, N-methyl-leucine, N-methyl-asparagine, N- methyl-lysine, N-methyl-aspartic acid, N-methyl-methionine, N-methyl-cysteine, N- methyl-phenylalanine, N-methyl-glutamic acid, N-methyl-threonine, N-methyl- glutamine, N-methyl-tryptophan, N-methyl-glycine, N-methyl-valine, N-methyl-
proline, N-methyl-serine, N-methyl-tyrosine, hydroxyproline, γ-carboxyglutamate, selinocysteine, O-phosphoserine, homoserine, norleucine, methionine sulfoxide, methionine methyl sulfonium, citrulline, ornithine, cysteine sulfonic acid, cysteine sulfinic acid, 3-aminoalanine, 3-dimethylaminoalanine, 2-amino-4- (dimethylamino)butanoic acid, 2,4-diaminobutanoic acid, 2-amino-6- (dimethylamino)hexanoic acid, 2-amino-5-(dimethylamino)pentanoic acid, and β- alanine, each independently as an L or D isomer.
115. The modified cell binding agent of any one of claims 105-113 wherein [XX] i_io is selected from the group consisting of: Val-Cit, Val-Lys, Phe-Lys, Lys-Lys, Ala-Lys, Phe-Cit, Leu-Cit, Lle-Cit, Trp, Cit, Phe-Ala, Phe-N9-tosyl-Arg, Phe-N9-nitro-Arg, Phe-Phe-Lys, D-Phe-Phe-Lys, Gly-Phe-Lys, Leu-Ala-Leu, Ile-Ala-Leu, Val-Ala-Val, Ala-Leu- Ala-Leu, β-Ala-Leu- Ala-Leu, Gly-Phe-Leu-Gly, Val-Arg, Arg-Val, Arg- Arg, Val-D-Cit, Val-D-Lys, Val-D-Arg, D-Val-Cit, D-Val-Lys, D- Val-Arg, D-Val-D- Cit, D-Val-D-Lys, D-Val-D-Arg, D-Arg-D-Arg, Ala- Ala, Ala-D-Ala, D-Ala-Ala, and D-Ala-D-Ala, Gly-Gly-Gly, Ala- Ala- Ala, D-Ala-Ala-Ala, Ala-D-Ala- Ala, Ala- Ala- D-Ala, Ala- Val-Cit, and Ala-Val-Ala.
116. The modified cell binding agent of any one of claims 105- 113, wherein [XX] MO is Gly-Gly-Gly, Gly-Gly-Ala, Val-Ala, Glu-Ala, or Glu(OMe)-Ala.
117. The modified cell binding agent of claim 105, wherein the modified cell binding agent is represented by one of the following formulas, or a salt thereof:
208
209
118. The modified cell binding agent of claim 117, wherein X" together with the adjacent -(C=0)- is a reactive ester; and Z is SRd, wherein Rd is pyridyl or nitropyridyl.
119. The modified cell binding agent of any one of claims 98-107 and 109-116, wherein U is represented by the following formula:
S3
wherein:
R3, R4, R5, R6, R7, Rs, Rio and Rn, for each occurrence, are independently H or an optionally substituted alkyl;
n, n', and n" are independently an integer from 1 to 10;
V is H, an optionally substituted alkyl, N02, or -NH-C(=0)-R301 ;
R301 is H or an optionally substituted alkyl;
R12 is H, an optionally substituted alkyl;
Q is H, a charged substituent or an ionizable group;
s3 is the site covalently linked the group hi; and
s4 is the site covalently linked to the group L2.
120. The modified cell binding agent of claim 119, wherein R3, R4, R5, R6, R7, R%, R10 and Rn are all H.
121. The modified cell binding agent of claim 119 or 120, wherein n, n', and n" are
independently an integer from 1 to 5.
122. The modified cell binding agent of claim 119, wherein U is represented by the
followin formula:
216
123. The modified cell binding agent of any one of claims 119 - 122, wherein V is H or N02.
124. A pharmaceutical composition comprising the conjugate of any one of claims 1 - 39 or the compound of any one of claims 40 - 71 ; and a pharmaceutically acceptable carrier.
125. A method of inhibiting abnormal cell growth or treating a proliferative disorder, an autoimmune disorder, destructive bone disorder, infectious disease, viral disease, fibrotic disease, neurodegenerative disorder, pancreatitis or kidney disease in a mammal comprising administering to said mammal a therapeutically effective amount of the conjugate of any one of claims 1 - 39, the compound of any one of claims 40 - 71, or the composition of claim 124; and, an optionally, a second therapeutic agent.
126. The method of claim 125, wherein said second therapeutic agent is administered to said mammal sequentially or consecutively.
127. The method of claim 125 or 126 wherein the method is for treating a condition
selected from the group consisting of cancer, rheumatoid arthritis, multiple sclerosis, graft versus host disease, transplant rejection, lupus, myositis, infection, and immune deficiency.
128. The method of claim 127, wherein the condition is cancer.
129. The method of claim 128, wherein the cancer is selected from the group consisting of breast cancer, colon cancer, brain cancer, prostate cancer, kidney cancer, pancreatic cancer, ovarian cancer, head and neck cancer, melanoma, colorectal cancer, gastric cancer, squamous cancer, small-cell lung cancer, non small-cell lung cancer, testicular cancer, Merkel cell carcinoma, glioblastoma, neuroblastoma, a cancer of a lymphatic organ, and a hematological malignancy.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361829893P | 2013-05-31 | 2013-05-31 | |
| US61/829,893 | 2013-05-31 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2014194030A2 true WO2014194030A2 (en) | 2014-12-04 |
| WO2014194030A3 WO2014194030A3 (en) | 2015-01-22 |
Family
ID=50983218
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2014/039919 WO2014194030A2 (en) | 2013-05-31 | 2014-05-29 | Conjugates comprising cell-binding agents and cytotoxic agents |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2014194030A2 (en) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106267225A (en) * | 2015-05-29 | 2017-01-04 | 上海新理念生物医药科技有限公司 | Three maleimide amine type connexon and application thereof |
| JP2019503979A (en) * | 2015-11-25 | 2019-02-14 | レゴケム バイオサイエンシズ, インク.Legochem Biosciences, Inc. | Complexes containing peptide groups and related methods |
| EP3380126A4 (en) * | 2015-11-25 | 2019-07-24 | LegoChem Biosciences, Inc. | Antibody-drug conjugates comprising branched linkers and methods related thereto |
| US10449258B2 (en) | 2015-06-09 | 2019-10-22 | Xdcexplorer (Shanghai) Co., Ltd. | Antibody drug conjugate, intermediate, preparation method, pharmaceutical composition and uses thereof |
| US10980890B2 (en) | 2014-05-28 | 2021-04-20 | Legochem Biosciences, Inc. | Compounds comprising self-immolative group |
| KR20220052465A (en) * | 2020-10-21 | 2022-04-28 | 순천향대학교 산학협력단 | Cell Penetrating Peptide-linker-anti-cancer drug and Pharmaceutical composition for prevention or treatment of colorectal cancer containing the same |
| US11345715B2 (en) | 2013-03-15 | 2022-05-31 | Regeneron Pharmaceuticals, Inc. | Biologically active molecules, conjugates thereof, and therapeutic uses |
| US11413353B2 (en) | 2015-11-25 | 2022-08-16 | Legochem Biosciences, Inc. | Conjugates comprising self-immolative groups and methods related thereto |
| US11446389B2 (en) | 2016-01-25 | 2022-09-20 | Regeneron Pharmaceuticals, Inc. | Maytansinoid derivatives, conjugates thereof, and methods of use |
| WO2022262516A1 (en) * | 2021-06-18 | 2022-12-22 | 北京海步医药科技有限公司 | Linker and conjugate therefor |
| US11596635B2 (en) | 2013-08-26 | 2023-03-07 | Regeneron Pharmaceuticals, Inc. | Pharmaceutical compositions comprising macrolide diastereomers, methods of their synthesis and therapeutic uses |
| US11654197B2 (en) | 2017-03-29 | 2023-05-23 | Legochem Biosciences, Inc. | Pyrrolobenzodiazepine dimer prodrug and ligand-linker conjugate compound of the same |
| US11707533B2 (en) | 2019-09-04 | 2023-07-25 | Legochem Biosciences, Inc. | Antibody-drug conjugate comprising antibody against human ROR1 and use for the same |
| US11827703B2 (en) | 2018-05-09 | 2023-11-28 | Legochem Biosciences, Inc. | Compositions and methods related to anti-CD19 antibody drug conjugates |
Citations (80)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1990005719A1 (en) | 1988-11-23 | 1990-05-31 | British Bio-Technology Limited | Hydroxamic acid based collagenase inhibitors |
| WO1992001047A1 (en) | 1990-07-10 | 1992-01-23 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| EP0606046A1 (en) | 1993-01-06 | 1994-07-13 | Ciba-Geigy Ag | Arylsulfonamido-substituted hydroxamic acids |
| JPH08175990A (en) | 1994-12-19 | 1996-07-09 | Mitsubishi Chem Corp | Pi3 kinase-inhibiting agent and its production |
| JPH08176070A (en) | 1994-12-19 | 1996-07-09 | Mitsubishi Chem Corp | Didepside derivative and pi3 kinase inhibitor |
| WO1996027583A1 (en) | 1995-03-08 | 1996-09-12 | Pfizer Inc. | Arylsulfonylamino hydroxamic acid derivatives |
| WO1996033172A1 (en) | 1995-04-20 | 1996-10-24 | Pfizer Inc. | Arylsulfonyl hydroxamic acid derivatives as mmp and tnf inhibitors |
| WO1997015658A1 (en) | 1995-10-26 | 1997-05-01 | Ludwig Institute For Cancer Research | Wortmannin phosphoinositide 3-kinase interaction site |
| US5639641A (en) | 1992-09-09 | 1997-06-17 | Immunogen Inc. | Resurfacing of rodent antibodies |
| EP0780386A1 (en) | 1995-12-20 | 1997-06-25 | F. Hoffmann-La Roche Ag | Matrix metalloprotease inhibitors |
| WO1997022596A1 (en) | 1995-12-18 | 1997-06-26 | Zeneca Limited | Quinazoline derivatives |
| WO1997030035A1 (en) | 1996-02-13 | 1997-08-21 | Zeneca Limited | Quinazoline derivatives as vegf inhibitors |
| WO1997032856A1 (en) | 1996-03-05 | 1997-09-12 | Zeneca Limited | 4-anilinoquinazoline derivatives |
| EP0818442A2 (en) | 1996-07-12 | 1998-01-14 | Pfizer Inc. | Cyclic sulphone derivatives as inhibitors of metalloproteinases and of the production of tumour necrosis factor |
| WO1998003516A1 (en) | 1996-07-18 | 1998-01-29 | Pfizer Inc. | Phosphinate based inhibitors of matrix metalloproteases |
| WO1998007697A1 (en) | 1996-08-23 | 1998-02-26 | Pfizer Inc. | Arylsulfonylamino hydroxamic acid derivatives |
| WO1998013354A1 (en) | 1996-09-25 | 1998-04-02 | Zeneca Limited | Quinazoline derivatives and pharmaceutical compositions containing them |
| WO1998030566A1 (en) | 1997-01-06 | 1998-07-16 | Pfizer Inc. | Cyclic sulfone derivatives |
| WO1998033768A1 (en) | 1997-02-03 | 1998-08-06 | Pfizer Products Inc. | Arylsulfonylamino hydroxamic acid derivatives |
| WO1998034918A1 (en) | 1997-02-11 | 1998-08-13 | Pfizer Inc. | Arylsulfonyl hydroxamic acid derivatives |
| WO1998034915A1 (en) | 1997-02-07 | 1998-08-13 | Pfizer Inc. | N-hydroxy-beta-sulfonyl-propionamide derivatives and their use as inhibitors of matrix metalloproteinases |
| WO1998035985A1 (en) | 1997-02-12 | 1998-08-20 | The Regents Of The University Of Michigan | Protein markers for lung cancer and use thereof |
| WO1999006587A2 (en) | 1997-08-01 | 1999-02-11 | Morphosys Ag | Novel method and phage for the identification of nucleic acid sequences encoding members of a multimeric (poly)peptide complex |
| WO1999007675A1 (en) | 1997-08-08 | 1999-02-18 | Pfizer Products Inc. | Aryloxyarylsulfonylamino hydroxamic acid derivatives |
| US5885793A (en) | 1991-12-02 | 1999-03-23 | Medical Research Council | Production of anti-self antibodies from antibody segment repertoires and displayed on phage |
| WO1999029667A1 (en) | 1997-12-05 | 1999-06-17 | Pfizer Limited | Hydroxamic acid derivatives as matrix metalloprotease (mmp) inhibitors |
| EP0931788A2 (en) | 1998-01-27 | 1999-07-28 | Pfizer Limited | Metalloprotease inhibitors |
| EP0945864A2 (en) | 1998-03-26 | 1999-09-29 | Sony Corporation | Selecting video material |
| WO1999052889A1 (en) | 1998-04-10 | 1999-10-21 | Pfizer Products Inc. | (4-arylsulfonylamino)-tetrahydropyran-4-carboxylic acid hydroxamides |
| WO1999052910A1 (en) | 1998-04-10 | 1999-10-21 | Pfizer Products Inc. | Bicyclic hydroxamic acid derivatives |
| EP1004578A2 (en) | 1998-11-05 | 2000-05-31 | Pfizer Products Inc. | 5-oxo-pyrrolidine-2-carboxylic acid hydroxamide derivatives |
| WO2000047212A1 (en) | 1999-02-10 | 2000-08-17 | Astrazeneca Ab | Quinazoline derivatives as angiogenesis inhibitors |
| WO2001032651A1 (en) | 1999-11-05 | 2001-05-10 | Astrazeneca Ab | Quinazoline derivatives as vegf inhibitors |
| WO2001060814A2 (en) | 2000-02-15 | 2001-08-23 | Sugen, Inc. | Pyrrole substituted 2-indolinone protein kinase inhibitors |
| JP2001247477A (en) | 2000-03-03 | 2001-09-11 | Teikoku Hormone Mfg Co Ltd | Antitumor medicine |
| WO2002020565A2 (en) | 2000-09-08 | 2002-03-14 | Universität Zürich | Collections of repeat proteins comprising repeat modules |
| US6403588B1 (en) | 2000-04-27 | 2002-06-11 | Yamanouchi Pharmaceutical Co., Ltd. | Imidazopyridine derivatives |
| US6441163B1 (en) | 2001-05-31 | 2002-08-27 | Immunogen, Inc. | Methods for preparation of cytotoxic conjugates of maytansinoids and cell binding agents |
| WO2003034997A2 (en) | 2001-10-24 | 2003-05-01 | Iconix Pharmaceuticals, Inc. | Modulators of phosphoinositide 3-kinase |
| WO2003035618A2 (en) | 2001-10-24 | 2003-05-01 | Iconix Pharmaceuticals, Inc. | Modulators of phosphoinositide 3-kinase |
| WO2003037886A2 (en) | 2001-10-30 | 2003-05-08 | Pharmacia Corporation | Heteroaromatic carboxamide derivatives for the treatment of inflammation |
| US6608056B1 (en) | 2000-04-27 | 2003-08-19 | Yamanouchi Pharmaceutical Co., Ltd | Fused heteroaryl derivatives |
| WO2004006916A1 (en) | 2002-07-10 | 2004-01-22 | Applied Research Systems Ars Holding Nv | Use of compounds for increasing spermatozoa motility |
| WO2004007491A1 (en) | 2002-07-10 | 2004-01-22 | Applied Research Systems Ars Holding N.V. | Azolidinone-vinyl fused-benzene derivatives |
| WO2004017950A2 (en) | 2002-08-22 | 2004-03-04 | Piramed Limited | Phosphadidylinositol 3,5-biphosphate inhibitors as anti-viral agents |
| US6703414B2 (en) | 2001-09-14 | 2004-03-09 | Arizona Board Of Regents On Behalf Of The University Of Arizona | Device and method for treating restenosis |
| US20040053946A1 (en) | 2001-01-16 | 2004-03-18 | Lackey Karen Elizabeth | Cancer treatment method |
| EP1417976A1 (en) | 2001-07-26 | 2004-05-12 | Santen Pharmaceutical Co., Ltd. | Remedy for glaucoma comprising as the active ingredient compound having pi3 kinase inhibitory effect |
| US20040092561A1 (en) | 2002-11-07 | 2004-05-13 | Thomas Ruckle | Azolidinone-vinyl fused -benzene derivatives |
| WO2006046040A1 (en) | 2004-10-25 | 2006-05-04 | Piramed Limited | Pharmaceutical compounds |
| WO2006083275A2 (en) | 2004-05-21 | 2006-08-10 | The Uab Research Foundation | Variable lymphocyte receptors, related polypeptides and nucleic acids, and uses thereof |
| US20060182750A1 (en) | 2005-02-11 | 2006-08-17 | Immunogen, Inc. | Process for preparing stable drug conjugates |
| US7101675B2 (en) | 1995-02-22 | 2006-09-05 | Yeda Research And Development Co. Ltd. | Modulators of regulatory proteins |
| US20070082365A1 (en) | 1998-12-10 | 2007-04-12 | Adnexus Therapeutics, Inc. | Protein scaffolds for antibody mimics and other binding proteins |
| WO2007042810A1 (en) | 2005-10-11 | 2007-04-19 | Ludwig Institute For Cancer Research | Pyrimidine derivatives for the treatment of cancer |
| WO2007062466A1 (en) | 2005-11-29 | 2007-06-07 | The University Of Sydney | Demibodies: dimerisation-activated therapeutic agents |
| US20070238667A1 (en) | 2006-03-03 | 2007-10-11 | Zongchao Jia | Compositions for treatment of cancer |
| WO2007147213A1 (en) | 2006-06-22 | 2007-12-27 | Walter And Eliza Hall Institute Of Medical Research | Structure of the insulin receptor ectodomain |
| US7342110B2 (en) | 2002-11-07 | 2008-03-11 | Immunogen Inc. | Anti-CD33 antibodies and method for treatment of acute myeloid leukemia using the same |
| US20080152586A1 (en) | 1992-09-25 | 2008-06-26 | Avipep Pty Limited | High avidity polyvalent and polyspecific reagents |
| US20080171040A1 (en) | 2004-06-01 | 2008-07-17 | Genentech, Inc. | Antibody-drug conjugates and methods |
| US20080305044A1 (en) | 2004-11-29 | 2008-12-11 | Seattle Genetics, Inc. | Engineered Antibodies and Immunoconjugates |
| US20090068738A1 (en) | 2004-11-01 | 2009-03-12 | The Regents Of The University Of California | Compositions and methods for modification of biomolecules |
| US7521541B2 (en) | 2004-09-23 | 2009-04-21 | Genetech Inc. | Cysteine engineered antibodies and conjugates |
| US7598375B2 (en) | 2005-08-09 | 2009-10-06 | Millenium Pharmaceuticals, Inc. | Method of acylating maytansinol with chiral amino acids |
| WO2009134977A1 (en) | 2008-04-30 | 2009-11-05 | Immunogen, Inc. | Cross-linkers and their uses |
| US7659241B2 (en) | 2002-07-31 | 2010-02-09 | Seattle Genetics, Inc. | Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease |
| US20100099649A1 (en) | 2008-10-03 | 2010-04-22 | Alexander Krantz | Site-specific chemical modification of proteins at their n-termini, enabling the formation of homogeneous adducts |
| US20100216708A1 (en) | 2008-10-31 | 2010-08-26 | Steven Jacobs | Fibronectin Type III Domain Based Scaffold Compositions, Methods and Uses |
| US20100255056A1 (en) | 2009-02-12 | 2010-10-07 | Steven Jacobs | Fibronectin Type III Domain Based Scaffold Compositions, Methods And Uses |
| US7811572B2 (en) | 2005-08-24 | 2010-10-12 | Immunogen, Inc. | Process for preparing purified drug conjugates |
| US20110003969A1 (en) | 2009-06-03 | 2011-01-06 | Immunogen Inc. | Conjugation methods |
| US20110118146A1 (en) | 2005-07-08 | 2011-05-19 | Daniel Steiner | Phage Display Using Cotranslational Translocation of Fusion Polypeptides |
| US20110224100A1 (en) | 2007-09-24 | 2011-09-15 | Fabio Parmeggiani | Designed armadillo repeat proteins |
| WO2011136645A1 (en) | 2010-04-27 | 2011-11-03 | Stichting Katholieke Universiteit, More Particularly Radboud University Nijmegen | Fused cyclooctyne compounds and their use in metal-free click reactions |
| US20110274623A1 (en) | 2010-04-30 | 2011-11-10 | Steven Jacobs | Stabilized Fibronectin Domain Compositions, Methods and Uses |
| US20120009181A1 (en) | 2010-02-24 | 2012-01-12 | Ab Olga | Folate Receptor 1 Antibodies and Immunoconjugates and Uses Thereof |
| US8163888B2 (en) | 2003-10-10 | 2012-04-24 | Immunogen, Inc. | Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates and methods of making said conjugates |
| US20120171115A1 (en) | 2009-12-23 | 2012-07-05 | Avipep Pty Limited | Immuno-conjugates and methods for producing them |
| US20120253021A1 (en) | 2011-03-29 | 2012-10-04 | Immunogen, Inc. | Preparation of maytansinoid antibody conjugates by a one-step process |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1997023243A1 (en) * | 1995-12-22 | 1997-07-03 | Bristol-Myers Squibb Company | Branched hydrazone linkers |
| CA2264610A1 (en) * | 1996-11-05 | 1998-05-14 | Bristol-Myers Squibb Company | Branched peptide linkers |
| US6777387B2 (en) * | 2000-03-31 | 2004-08-17 | Enzon Pharmaceuticals, Inc. | Terminally-branched polymeric linkers containing extension moieties and polymeric conjugates containing the same |
| EP2800586A1 (en) * | 2012-01-03 | 2014-11-12 | Invictus Oncology Pvt. Ltd. | Ligand-targeted molecules and methods thereof |
| NO2789793T3 (en) * | 2012-10-24 | 2018-01-27 |
-
2014
- 2014-05-29 WO PCT/US2014/039919 patent/WO2014194030A2/en active Application Filing
Patent Citations (99)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1990005719A1 (en) | 1988-11-23 | 1990-05-31 | British Bio-Technology Limited | Hydroxamic acid based collagenase inhibitors |
| WO1992001047A1 (en) | 1990-07-10 | 1992-01-23 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| US5969108A (en) | 1990-07-10 | 1999-10-19 | Medical Research Council | Methods for producing members of specific binding pairs |
| US5885793A (en) | 1991-12-02 | 1999-03-23 | Medical Research Council | Production of anti-self antibodies from antibody segment repertoires and displayed on phage |
| US5639641A (en) | 1992-09-09 | 1997-06-17 | Immunogen Inc. | Resurfacing of rodent antibodies |
| US20080152586A1 (en) | 1992-09-25 | 2008-06-26 | Avipep Pty Limited | High avidity polyvalent and polyspecific reagents |
| EP0606046A1 (en) | 1993-01-06 | 1994-07-13 | Ciba-Geigy Ag | Arylsulfonamido-substituted hydroxamic acids |
| JPH08175990A (en) | 1994-12-19 | 1996-07-09 | Mitsubishi Chem Corp | Pi3 kinase-inhibiting agent and its production |
| JPH08176070A (en) | 1994-12-19 | 1996-07-09 | Mitsubishi Chem Corp | Didepside derivative and pi3 kinase inhibitor |
| US7101675B2 (en) | 1995-02-22 | 2006-09-05 | Yeda Research And Development Co. Ltd. | Modulators of regulatory proteins |
| WO1996027583A1 (en) | 1995-03-08 | 1996-09-12 | Pfizer Inc. | Arylsulfonylamino hydroxamic acid derivatives |
| US5863949A (en) | 1995-03-08 | 1999-01-26 | Pfizer Inc | Arylsulfonylamino hydroxamic acid derivatives |
| WO1996033172A1 (en) | 1995-04-20 | 1996-10-24 | Pfizer Inc. | Arylsulfonyl hydroxamic acid derivatives as mmp and tnf inhibitors |
| US5861510A (en) | 1995-04-20 | 1999-01-19 | Pfizer Inc | Arylsulfonyl hydroxamic acid derivatives as MMP and TNF inhibitors |
| WO1997015658A1 (en) | 1995-10-26 | 1997-05-01 | Ludwig Institute For Cancer Research | Wortmannin phosphoinositide 3-kinase interaction site |
| WO1997022596A1 (en) | 1995-12-18 | 1997-06-26 | Zeneca Limited | Quinazoline derivatives |
| EP0780386A1 (en) | 1995-12-20 | 1997-06-25 | F. Hoffmann-La Roche Ag | Matrix metalloprotease inhibitors |
| WO1997030035A1 (en) | 1996-02-13 | 1997-08-21 | Zeneca Limited | Quinazoline derivatives as vegf inhibitors |
| WO1997032856A1 (en) | 1996-03-05 | 1997-09-12 | Zeneca Limited | 4-anilinoquinazoline derivatives |
| EP0818442A2 (en) | 1996-07-12 | 1998-01-14 | Pfizer Inc. | Cyclic sulphone derivatives as inhibitors of metalloproteinases and of the production of tumour necrosis factor |
| WO1998003516A1 (en) | 1996-07-18 | 1998-01-29 | Pfizer Inc. | Phosphinate based inhibitors of matrix metalloproteases |
| WO1998007697A1 (en) | 1996-08-23 | 1998-02-26 | Pfizer Inc. | Arylsulfonylamino hydroxamic acid derivatives |
| WO1998013354A1 (en) | 1996-09-25 | 1998-04-02 | Zeneca Limited | Quinazoline derivatives and pharmaceutical compositions containing them |
| WO1998030566A1 (en) | 1997-01-06 | 1998-07-16 | Pfizer Inc. | Cyclic sulfone derivatives |
| WO1998033768A1 (en) | 1997-02-03 | 1998-08-06 | Pfizer Products Inc. | Arylsulfonylamino hydroxamic acid derivatives |
| WO1998034915A1 (en) | 1997-02-07 | 1998-08-13 | Pfizer Inc. | N-hydroxy-beta-sulfonyl-propionamide derivatives and their use as inhibitors of matrix metalloproteinases |
| WO1998034918A1 (en) | 1997-02-11 | 1998-08-13 | Pfizer Inc. | Arylsulfonyl hydroxamic acid derivatives |
| WO1998035985A1 (en) | 1997-02-12 | 1998-08-20 | The Regents Of The University Of Michigan | Protein markers for lung cancer and use thereof |
| WO1999006587A2 (en) | 1997-08-01 | 1999-02-11 | Morphosys Ag | Novel method and phage for the identification of nucleic acid sequences encoding members of a multimeric (poly)peptide complex |
| WO1999007675A1 (en) | 1997-08-08 | 1999-02-18 | Pfizer Products Inc. | Aryloxyarylsulfonylamino hydroxamic acid derivatives |
| WO1999029667A1 (en) | 1997-12-05 | 1999-06-17 | Pfizer Limited | Hydroxamic acid derivatives as matrix metalloprotease (mmp) inhibitors |
| EP0931788A2 (en) | 1998-01-27 | 1999-07-28 | Pfizer Limited | Metalloprotease inhibitors |
| EP0945864A2 (en) | 1998-03-26 | 1999-09-29 | Sony Corporation | Selecting video material |
| WO1999052889A1 (en) | 1998-04-10 | 1999-10-21 | Pfizer Products Inc. | (4-arylsulfonylamino)-tetrahydropyran-4-carboxylic acid hydroxamides |
| WO1999052910A1 (en) | 1998-04-10 | 1999-10-21 | Pfizer Products Inc. | Bicyclic hydroxamic acid derivatives |
| EP1004578A2 (en) | 1998-11-05 | 2000-05-31 | Pfizer Products Inc. | 5-oxo-pyrrolidine-2-carboxylic acid hydroxamide derivatives |
| US20070082365A1 (en) | 1998-12-10 | 2007-04-12 | Adnexus Therapeutics, Inc. | Protein scaffolds for antibody mimics and other binding proteins |
| US20080139791A1 (en) | 1998-12-10 | 2008-06-12 | Adnexus Therapeutics, Inc. | Pharmaceutically acceptable Fn3 Polypeptides for human treatments |
| WO2000047212A1 (en) | 1999-02-10 | 2000-08-17 | Astrazeneca Ab | Quinazoline derivatives as angiogenesis inhibitors |
| WO2001032651A1 (en) | 1999-11-05 | 2001-05-10 | Astrazeneca Ab | Quinazoline derivatives as vegf inhibitors |
| WO2001060814A2 (en) | 2000-02-15 | 2001-08-23 | Sugen, Inc. | Pyrrole substituted 2-indolinone protein kinase inhibitors |
| JP2001247477A (en) | 2000-03-03 | 2001-09-11 | Teikoku Hormone Mfg Co Ltd | Antitumor medicine |
| US6770641B2 (en) | 2000-04-27 | 2004-08-03 | Yamanouchi Pharmaceutical Co., Ltd. | Fused heteroaryl derivatives |
| US7037915B2 (en) | 2000-04-27 | 2006-05-02 | Astellas Pharma Inc. | Fused heteroaryl derivatives |
| US6838457B2 (en) | 2000-04-27 | 2005-01-04 | Yamanouchi Pharmaceutical Co., Ltd. | Fused heteroaryl derivatives |
| US6608056B1 (en) | 2000-04-27 | 2003-08-19 | Yamanouchi Pharmaceutical Co., Ltd | Fused heteroaryl derivatives |
| US6608053B2 (en) | 2000-04-27 | 2003-08-19 | Yamanouchi Pharmaceutical Co., Ltd. | Fused heteroaryl derivatives |
| US7173029B2 (en) | 2000-04-27 | 2007-02-06 | Astellas Pharma Inc. | Fused heteroaryl derivatives |
| US6653320B2 (en) | 2000-04-27 | 2003-11-25 | Yamanouchi Pharmaceutical Co. Ltd. | Imidazopyridine derivatives |
| US6403588B1 (en) | 2000-04-27 | 2002-06-11 | Yamanouchi Pharmaceutical Co., Ltd. | Imidazopyridine derivatives |
| WO2002020565A2 (en) | 2000-09-08 | 2002-03-14 | Universität Zürich | Collections of repeat proteins comprising repeat modules |
| US20090082274A1 (en) | 2000-09-08 | 2009-03-26 | Universitat Zurich | Collections of repeat proteins comprising repeat modules |
| US20040132028A1 (en) | 2000-09-08 | 2004-07-08 | Stumpp Michael Tobias | Collection of repeat proteins comprising repeat modules |
| US20040053946A1 (en) | 2001-01-16 | 2004-03-18 | Lackey Karen Elizabeth | Cancer treatment method |
| US7368565B2 (en) | 2001-05-31 | 2008-05-06 | Immunogen Inc. | Methods for preparation of cytotoxic conjugates of maytansinoids and cell binding agents |
| US6441163B1 (en) | 2001-05-31 | 2002-08-27 | Immunogen, Inc. | Methods for preparation of cytotoxic conjugates of maytansinoids and cell binding agents |
| EP1417976A1 (en) | 2001-07-26 | 2004-05-12 | Santen Pharmaceutical Co., Ltd. | Remedy for glaucoma comprising as the active ingredient compound having pi3 kinase inhibitory effect |
| US6703414B2 (en) | 2001-09-14 | 2004-03-09 | Arizona Board Of Regents On Behalf Of The University Of Arizona | Device and method for treating restenosis |
| US20030158212A1 (en) | 2001-10-24 | 2003-08-21 | Teri Melese | Modulators of phosphoinositide 3-kinase |
| WO2003035618A2 (en) | 2001-10-24 | 2003-05-01 | Iconix Pharmaceuticals, Inc. | Modulators of phosphoinositide 3-kinase |
| US20030149074A1 (en) | 2001-10-24 | 2003-08-07 | Teri Melese | Modulators of phosphoinositide 3-kinase |
| WO2003034997A2 (en) | 2001-10-24 | 2003-05-01 | Iconix Pharmaceuticals, Inc. | Modulators of phosphoinositide 3-kinase |
| WO2003037886A2 (en) | 2001-10-30 | 2003-05-08 | Pharmacia Corporation | Heteroaromatic carboxamide derivatives for the treatment of inflammation |
| WO2004006916A1 (en) | 2002-07-10 | 2004-01-22 | Applied Research Systems Ars Holding Nv | Use of compounds for increasing spermatozoa motility |
| WO2004007491A1 (en) | 2002-07-10 | 2004-01-22 | Applied Research Systems Ars Holding N.V. | Azolidinone-vinyl fused-benzene derivatives |
| US7659241B2 (en) | 2002-07-31 | 2010-02-09 | Seattle Genetics, Inc. | Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease |
| WO2004017950A2 (en) | 2002-08-22 | 2004-03-04 | Piramed Limited | Phosphadidylinositol 3,5-biphosphate inhibitors as anti-viral agents |
| US7557189B2 (en) | 2002-11-07 | 2009-07-07 | Immunogen Inc. | Anti-CD33 antibodies and method for treatment of acute myeloid leukemia using the same |
| US20040092561A1 (en) | 2002-11-07 | 2004-05-13 | Thomas Ruckle | Azolidinone-vinyl fused -benzene derivatives |
| US7342110B2 (en) | 2002-11-07 | 2008-03-11 | Immunogen Inc. | Anti-CD33 antibodies and method for treatment of acute myeloid leukemia using the same |
| US8163888B2 (en) | 2003-10-10 | 2012-04-24 | Immunogen, Inc. | Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates and methods of making said conjugates |
| WO2006083275A2 (en) | 2004-05-21 | 2006-08-10 | The Uab Research Foundation | Variable lymphocyte receptors, related polypeptides and nucleic acids, and uses thereof |
| US20080171040A1 (en) | 2004-06-01 | 2008-07-17 | Genentech, Inc. | Antibody-drug conjugates and methods |
| US7521541B2 (en) | 2004-09-23 | 2009-04-21 | Genetech Inc. | Cysteine engineered antibodies and conjugates |
| WO2006046031A1 (en) | 2004-10-25 | 2006-05-04 | Piramed Limited | Pharmaceutical compounds |
| WO2006046035A1 (en) | 2004-10-25 | 2006-05-04 | Piramed Limited | Pharmaceutical compounds |
| WO2006046040A1 (en) | 2004-10-25 | 2006-05-04 | Piramed Limited | Pharmaceutical compounds |
| US20090068738A1 (en) | 2004-11-01 | 2009-03-12 | The Regents Of The University Of California | Compositions and methods for modification of biomolecules |
| US20080305044A1 (en) | 2004-11-29 | 2008-12-11 | Seattle Genetics, Inc. | Engineered Antibodies and Immunoconjugates |
| US20060182750A1 (en) | 2005-02-11 | 2006-08-17 | Immunogen, Inc. | Process for preparing stable drug conjugates |
| US20110118146A1 (en) | 2005-07-08 | 2011-05-19 | Daniel Steiner | Phage Display Using Cotranslational Translocation of Fusion Polypeptides |
| US7598375B2 (en) | 2005-08-09 | 2009-10-06 | Millenium Pharmaceuticals, Inc. | Method of acylating maytansinol with chiral amino acids |
| US7811572B2 (en) | 2005-08-24 | 2010-10-12 | Immunogen, Inc. | Process for preparing purified drug conjugates |
| WO2007042806A1 (en) | 2005-10-11 | 2007-04-19 | Ludwig Institute For Cancer Research | Pyrimidine derivatives for the treatment of cancer |
| WO2007042810A1 (en) | 2005-10-11 | 2007-04-19 | Ludwig Institute For Cancer Research | Pyrimidine derivatives for the treatment of cancer |
| WO2007062466A1 (en) | 2005-11-29 | 2007-06-07 | The University Of Sydney | Demibodies: dimerisation-activated therapeutic agents |
| US20070238667A1 (en) | 2006-03-03 | 2007-10-11 | Zongchao Jia | Compositions for treatment of cancer |
| WO2007147213A1 (en) | 2006-06-22 | 2007-12-27 | Walter And Eliza Hall Institute Of Medical Research | Structure of the insulin receptor ectodomain |
| US20110224100A1 (en) | 2007-09-24 | 2011-09-15 | Fabio Parmeggiani | Designed armadillo repeat proteins |
| WO2009134977A1 (en) | 2008-04-30 | 2009-11-05 | Immunogen, Inc. | Cross-linkers and their uses |
| US20100099649A1 (en) | 2008-10-03 | 2010-04-22 | Alexander Krantz | Site-specific chemical modification of proteins at their n-termini, enabling the formation of homogeneous adducts |
| US20100216708A1 (en) | 2008-10-31 | 2010-08-26 | Steven Jacobs | Fibronectin Type III Domain Based Scaffold Compositions, Methods and Uses |
| US20100255056A1 (en) | 2009-02-12 | 2010-10-07 | Steven Jacobs | Fibronectin Type III Domain Based Scaffold Compositions, Methods And Uses |
| US20110003969A1 (en) | 2009-06-03 | 2011-01-06 | Immunogen Inc. | Conjugation methods |
| US20120171115A1 (en) | 2009-12-23 | 2012-07-05 | Avipep Pty Limited | Immuno-conjugates and methods for producing them |
| US20120009181A1 (en) | 2010-02-24 | 2012-01-12 | Ab Olga | Folate Receptor 1 Antibodies and Immunoconjugates and Uses Thereof |
| WO2011136645A1 (en) | 2010-04-27 | 2011-11-03 | Stichting Katholieke Universiteit, More Particularly Radboud University Nijmegen | Fused cyclooctyne compounds and their use in metal-free click reactions |
| US20110274623A1 (en) | 2010-04-30 | 2011-11-10 | Steven Jacobs | Stabilized Fibronectin Domain Compositions, Methods and Uses |
| US20120253021A1 (en) | 2011-03-29 | 2012-10-04 | Immunogen, Inc. | Preparation of maytansinoid antibody conjugates by a one-step process |
Non-Patent Citations (56)
| Title |
|---|
| "The Chemistry of Heterocyclic Compounds, A Series of Monographs", vol. 13, 1950, JOHN WILEY & SONS |
| ANGEW CHEM. INTL. ED. ENGL., vol. 33, 1994, pages 183 - 186 |
| ANGEW. CHEM. INT. ED., vol. 45, no. 32, 2006, pages 5307 |
| BINZ, H.K.; AMSTUTZ, P.; PLUCKTHUN, A., NATURE BIOTECHNOLOGY, vol. 23, 2005, pages 1257 - 1268 |
| BOEGGEMAN E ET AL., BIOCONJUG CHEM., 20 June 2009 (2009-06-20), pages 1228 - 36 |
| BOUVIER ET AL., METH. ENZYMOL., vol. 248, 1995, pages 614 |
| C. ZAHND ET AL., CANCER RES., vol. 70, 2010, pages 1595 - 1605 |
| CARTER, NATURE REVS., vol. 6, 2006, pages 343 - 357 |
| CHEM. BIOL., vol. 2, no. 4, 2007, pages 247 |
| CHENOWETH, K. ET AL., ORGANIC & BIOMOLECULAR CHEMISTRY, 2009, pages 5255 |
| DANE ET AL., MOL. CANCER. THER., vol. 8, no. 5, 2009, pages 1312 - 1318 |
| DAVIS L.K. ET AL., J. AM. CHEM. SOC., vol. 134, 2012, pages 10317 - 10320 |
| DEBETS, M. F. ET AL., ACC. CHEM. RES., vol. 44, no. 9, 2011, pages 805 - 815 |
| DUBE, D. H. ET AL., PROC. NATL. ACAD. SCI. USA, vol. 103, 2006, pages 4819 - 4824 |
| DUNN ET AL., METH. ENZYMOL., vol. 241, 1994, pages 254 |
| GLASSNER, M. ET AL., J. AM. CHEM. SOC., vol. 134, 2012, pages 7274 - 7277 |
| GOFF; CARROLL, BIOCONJUGATE CHEM., vol. 1, no. 6, 1990, pages 381 - 386 |
| GOLD B. ET AL., J. AM. CHEM. SOC., vol. 135, no. 4, 2013, pages 1558 - 1569 |
| GREG T. HERMANSON: "Bioconjugate Techniques, 2nd ed.", 2008, ACADEMIC PRESS, INC. |
| HANG, H. C. ET AL., PROC. NATL. ACAD. SCI. USA, vol. 100, 2003, pages 14846 - 14851 |
| HANSELL, C. F. ET AL., J. AM. CHEM. SOC., vol. 133, 2011, pages 13828 - 13831 |
| J. AM. CHEM. SOC., vol. 130, no. 35, 2008, pages 11762 |
| J. AM. CHEM. SOC., vol. 134, 2012, pages 9199 - 9208 |
| J. AM. CHEM. SOC., vol. 82, 1960, pages 5566 |
| J.D. GRIFFIN ET AL., LEUKEMIA RES., vol. 8, 1984, pages 521 |
| JEGER S ET AL., ANGEW CHEM INT ED ENGL., vol. 49, no. 51, 17 December 2010 (2010-12-17), pages 9995 - 9997 |
| JEWETT, J.C. ET AL., J. AM. CHEM. SOC., vol. 132, no. 11, 2010, pages 3688 - 3690 |
| KIM ET AL., MOL. CANCER THER., vol. 7, 2008, pages 2486 - 2497 |
| LANG K. ET AL., J. AM. CHEM. SOC., vol. 134, 2012, pages 10317 - 10320 |
| MAHAL, L. K. ET AL., SCIENCE, vol. 276, 1997, pages 1125 - 1128 |
| MASTERS ET AL.: "AMYLOID PROTEIN PRECURSOR IN DEVELOPMENT, AGING, AND ALZHEIMER'S DISEASE", 1994, article HARDY ET AL., pages: 190 - 198 |
| MATAYOSHI ET AL., SCIENCE, vol. 247, 1990, pages 954 |
| NEAL K. DEVARAJ, SYNLETT, vol. 23, no. 15, 2012, pages 2147 - 2152 |
| NISONOFF, AL., ARCH. BIOCHEM. BIOPHYS, vol. 89, 1960, pages 230 - 244 |
| O'KEEFE ET AL., J. BIOL. CHEM., vol. 260, 1985, pages 932 - 937 |
| PAQUETTE, LEO A.: "Principles of Modem Heterocyclic Chemistry", 1968, W. A. BENJAMIN, article "Chapters 1, 3, 4, 6, 7, and 9" |
| PARHAM, J. IMMUNOL., vol. 131, 1983, pages 2895 - 2902 |
| PRESCHER, J. A. ET AL., NATURE, vol. 430, 2004, pages 873 - 877 |
| R. KONTERMANN; S. DUBEL: "Antibody Engineering", 2001, SPRINGER-VERLAG |
| SAXON, E.; BERTOZZI, C. R., SCIENCE, vol. 287, 2000, pages 2007 - 2010 |
| SEIDAH ET AL., METH. ENZYMOL., vol. 244, 1994, pages 175 |
| SEITCHIK J.L. ET AL., J. AM. CHEM. SOC., vol. 134, no. 6, 2012, pages 2898 - 2901 |
| SLETTEN E.M. ET AL., ANGEW CHEM. INT. ED. ENGL., vol. 48, no. 38, 2009, pages 6974 - 6998 |
| SMITH ET AL., METH. ENZYMOL., vol. 244, 1994, pages 412 |
| SPRING ET AL., J. IMMUNOL., vol. 113, 1974, pages 470 - 478 |
| STAN AC ET AL., CANCER RES., vol. 59, no. 1, 1 January 1999 (1999-01-01), pages 115 - 21 |
| THORNBERRY, METH. ENZYMOL., vol. 244, 1994, pages 615 |
| VOCADLO, D. J. ET AL., PROC. NATL. ACAD. SCI. USA, vol. 100, 2003, pages 9116 - 9121 |
| VU HONG ET AL., BIOCONJUGATE CHEM., vol. 21, no. 10, 2010, pages 1912 - 1916 |
| WANG ET AL., PROC. NATL. ACAD. SCI. USA, vol. 108, no. 17, 2011, pages 6909 - 6914 |
| WEBER ET AL., METH. ENZYMOL., vol. 244, 1994, pages 595 |
| WIDDISON, W. C. ET AL.: "Semisynthetic maytansine analogues for the targeted treatment of cancer", J. MED. CHEM., vol. 49, no. 14, 2006, pages 4392 - 408 |
| WIDDISON, W. C. ET AL.: "Semisynthetic maytansine analogues for the targeted treatment of cancer", J. MED. CHEM., vol. 49, no. 14, 2006, pages 4392 - 4408 |
| YAGUCHI ET AL., JOUR. OF THE NAT. CANCER INST., vol. 98, no. 8, 2006, pages 545 - 556 |
| ZAHND ET AL., J. BIOL. CHEM., vol. 281, no. 46, 2006, pages 35167 - 35175 |
| ZUCKER S., CURRENT TOPICS IN DEVELOPMENTAL BIOLOGY: CELL SURFACE PROTEASES, vol. 54, 2003 |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11345715B2 (en) | 2013-03-15 | 2022-05-31 | Regeneron Pharmaceuticals, Inc. | Biologically active molecules, conjugates thereof, and therapeutic uses |
| US11596635B2 (en) | 2013-08-26 | 2023-03-07 | Regeneron Pharmaceuticals, Inc. | Pharmaceutical compositions comprising macrolide diastereomers, methods of their synthesis and therapeutic uses |
| US10980890B2 (en) | 2014-05-28 | 2021-04-20 | Legochem Biosciences, Inc. | Compounds comprising self-immolative group |
| CN106267225B (en) * | 2015-05-29 | 2020-03-06 | 上海新理念生物医药科技有限公司 | Trimaleimide-type linker and use thereof |
| CN106267225A (en) * | 2015-05-29 | 2017-01-04 | 上海新理念生物医药科技有限公司 | Three maleimide amine type connexon and application thereof |
| US10449258B2 (en) | 2015-06-09 | 2019-10-22 | Xdcexplorer (Shanghai) Co., Ltd. | Antibody drug conjugate, intermediate, preparation method, pharmaceutical composition and uses thereof |
| JP7120765B2 (en) | 2015-11-25 | 2022-08-17 | レゴケム バイオサイエンシズ, インク. | CONJUGATES CONTAINING PEPTIDE GROUPS AND RELATED METHODS |
| AU2016359234B2 (en) * | 2015-11-25 | 2022-09-08 | Legochem Biosciences, Inc. | Conjugates comprising peptide groups and methods related thereto |
| US11173214B2 (en) | 2015-11-25 | 2021-11-16 | Legochem Biosciences, Inc. | Antibody-drug conjugates comprising branched linkers and methods related thereto |
| JP2019503979A (en) * | 2015-11-25 | 2019-02-14 | レゴケム バイオサイエンシズ, インク.Legochem Biosciences, Inc. | Complexes containing peptide groups and related methods |
| EP3380126A4 (en) * | 2015-11-25 | 2019-07-24 | LegoChem Biosciences, Inc. | Antibody-drug conjugates comprising branched linkers and methods related thereto |
| US11413353B2 (en) | 2015-11-25 | 2022-08-16 | Legochem Biosciences, Inc. | Conjugates comprising self-immolative groups and methods related thereto |
| EP3380125A4 (en) * | 2015-11-25 | 2019-08-28 | LegoChem Biosciences, Inc. | Conjugates comprising peptide groups and methods related thereto |
| US11167040B2 (en) | 2015-11-25 | 2021-11-09 | Legochem Biosciences, Inc. | Conjugates comprising peptide groups and methods related thereto |
| US11446389B2 (en) | 2016-01-25 | 2022-09-20 | Regeneron Pharmaceuticals, Inc. | Maytansinoid derivatives, conjugates thereof, and methods of use |
| US11654197B2 (en) | 2017-03-29 | 2023-05-23 | Legochem Biosciences, Inc. | Pyrrolobenzodiazepine dimer prodrug and ligand-linker conjugate compound of the same |
| US11827703B2 (en) | 2018-05-09 | 2023-11-28 | Legochem Biosciences, Inc. | Compositions and methods related to anti-CD19 antibody drug conjugates |
| US11707533B2 (en) | 2019-09-04 | 2023-07-25 | Legochem Biosciences, Inc. | Antibody-drug conjugate comprising antibody against human ROR1 and use for the same |
| KR102465431B1 (en) | 2020-10-21 | 2022-11-09 | 순천향대학교 산학협력단 | Cell Penetrating Peptide-linker-anti-cancer drug and Pharmaceutical composition for prevention or treatment of colorectal cancer containing the same |
| KR20220052465A (en) * | 2020-10-21 | 2022-04-28 | 순천향대학교 산학협력단 | Cell Penetrating Peptide-linker-anti-cancer drug and Pharmaceutical composition for prevention or treatment of colorectal cancer containing the same |
| WO2022262516A1 (en) * | 2021-06-18 | 2022-12-22 | 北京海步医药科技有限公司 | Linker and conjugate therefor |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2014194030A3 (en) | 2015-01-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2014194030A2 (en) | Conjugates comprising cell-binding agents and cytotoxic agents | |
| US9999680B2 (en) | Conjugates comprising cell-binding agents and maytansinoids as cytotoxic agents | |
| US10570212B2 (en) | Cytotoxic benzodiazepine derivatives | |
| US9624240B2 (en) | Maytansinoid derivatives with sulfoxide linker | |
| US9617270B2 (en) | Cytotoxic benzodiazepine derivatives | |
| US20180355055A1 (en) | Novel benzodiazepine derivatives | |
| WO2014134457A2 (en) | Conjugates comprising cell-binding agents and cytotoxic agents | |
| EP2961435B1 (en) | Conjugates comprising cell-binding agents and cytotoxic agents | |
| US20130029900A1 (en) | Novel maytansinoid derivatives with peptide linker and conjugates thereof | |
| IL290698B2 (en) | Maytansinoid derivatives with self-immolative peptide linkers and conjugates thereof | |
| USRE49918E1 (en) | Cytotoxic benzodiazepine derivatives | |
| AU2019206132A1 (en) | Cytotoxic benzodiazepine derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14732781 Country of ref document: EP Kind code of ref document: A2 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14732781 Country of ref document: EP Kind code of ref document: A2 |