US6368356B1 - Medical devices comprising hydrogel polymers having improved mechanical properties - Google Patents
Medical devices comprising hydrogel polymers having improved mechanical properties Download PDFInfo
- Publication number
- US6368356B1 US6368356B1 US09/512,698 US51269800A US6368356B1 US 6368356 B1 US6368356 B1 US 6368356B1 US 51269800 A US51269800 A US 51269800A US 6368356 B1 US6368356 B1 US 6368356B1
- Authority
- US
- United States
- Prior art keywords
- medical device
- polymer
- segment
- crosslinked
- stent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 *N(OCP)C(=O)NCNC(=O)N(*)C(=O)P.*N=C=NC/N=C(\N*)OC(=O)P.*N=C=NC/N=C(\O)N(*)C(=O)P.*N=C=NCN=C=N*.*N=C=NCNC(=O)N(*)C(=O)P.O=C(O)P.O=C(O)P Chemical compound *N(OCP)C(=O)NCNC(=O)N(*)C(=O)P.*N=C=NC/N=C(\N*)OC(=O)P.*N=C=NC/N=C(\O)N(*)C(=O)P.*N=C=NCN=C=N*.*N=C=NCNC(=O)N(*)C(=O)P.O=C(O)P.O=C(O)P 0.000 description 1
- OBOUBBNKYRXYIR-UHFFFAOYSA-N C1CN1CN1CC1.CC.CC.CC.CC.CC.CC(=O)N(CCO)CN(CCO)C(C)=O.CC(=O)N(CCO)CN1CC1.CC(=O)O.CC(=O)O.CC(=O)OCCNCN1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1 Chemical compound C1CN1CN1CC1.CC.CC.CC.CC.CC.CC(=O)N(CCO)CN(CCO)C(C)=O.CC(=O)N(CCO)CN1CC1.CC(=O)O.CC(=O)O.CC(=O)OCCNCN1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1.CC1CC1 OBOUBBNKYRXYIR-UHFFFAOYSA-N 0.000 description 1
- VTWAWMLOUJACQE-UHFFFAOYSA-N CCCOC(=O)CCN1CC1 Chemical compound CCCOC(=O)CCN1CC1 VTWAWMLOUJACQE-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M27/00—Drainage appliance for wounds or the like, i.e. wound drains, implanted drains
- A61M27/002—Implant devices for drainage of body fluids from one part of the body to another
- A61M27/008—Implant devices for drainage of body fluids from one part of the body to another pre-shaped, for use in the urethral or ureteral tract
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/52—Hydrogels or hydrocolloids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/14—Materials characterised by their function or physical properties, e.g. lubricating compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/14—Materials characterised by their function or physical properties, e.g. lubricating compositions
- A61L29/145—Hydrogels or hydrocolloids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/145—Hydrogels or hydrocolloids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/18—Materials at least partially X-ray or laser opaque
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08B—POLYSACCHARIDES; DERIVATIVES THEREOF
- C08B37/00—Preparation of polysaccharides not provided for in groups C08B1/00 - C08B35/00; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08B—POLYSACCHARIDES; DERIVATIVES THEREOF
- C08B37/00—Preparation of polysaccharides not provided for in groups C08B1/00 - C08B35/00; Derivatives thereof
- C08B37/006—Heteroglycans, i.e. polysaccharides having more than one sugar residue in the main chain in either alternating or less regular sequence; Gellans; Succinoglycans; Arabinogalactans; Tragacanth or gum tragacanth or traganth from Astragalus; Gum Karaya from Sterculia urens; Gum Ghatti from Anogeissus latifolia; Derivatives thereof
- C08B37/0084—Guluromannuronans, e.g. alginic acid, i.e. D-mannuronic acid and D-guluronic acid units linked with alternating alpha- and beta-1,4-glycosidic bonds; Derivatives thereof, e.g. alginates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J3/00—Processes of treating or compounding macromolecular substances
- C08J3/24—Crosslinking, e.g. vulcanising, of macromolecules
- C08J3/243—Two or more independent types of crosslinking for one or more polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2300/00—Characterised by the use of unspecified polymers
- C08J2300/14—Water soluble or water swellable polymers, e.g. aqueous gels
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S524/00—Synthetic resins or natural rubbers -- part of the class 520 series
- Y10S524/918—Wood patching composition
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S525/00—Synthetic resins or natural rubbers -- part of the class 520 series
- Y10S525/903—Interpenetrating network
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S623/00—Prosthesis, i.e. artificial body members, parts thereof, or aids and accessories therefor
- Y10S623/924—Material characteristic
- Y10S623/925—Natural
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S623/00—Prosthesis, i.e. artificial body members, parts thereof, or aids and accessories therefor
- Y10S623/924—Material characteristic
- Y10S623/926—Synthetic
Definitions
- This invention relates to shaped medical devices comprising polymer hydrogels having improved mechanical properties. More particularly, the invention relates to shaped medical devices which can function as temporary implants which do not require additional surgical procedures for removal.
- Shaped medical devices for insertion and/or implantation into the body have a variety of applications including drug delivery, tissue engineering, vascular surgery (e.g., angioplasty) and drainage (e.g., from the kidney).
- Such devices include vascular grafts, stents, catheters, cannulas, plugs, constrictors, tissue scaffolds, and tissue or biological encapsulants, and the like.
- Typical stents are permanent devices which require a surgical procedure to remove and may cause discomfort.
- Shaped medical devices have also been disclosed which are made from biodegradable polymers. These remain stable in vivo for a period of time but eventually biodegrade into small fragments which are removed by the body by normal elimination in the urine or feces. In the case of ureteral stents, the fragmentation of such biodegradeable polymers into small pieces can obstruct the ureter and cause patient discomfort.
- Typical biodegradable polymers used in these devices include polyesters, polyanhydrides and polyorthoesters which undergo hydrolytic chain cleavage, as disclosed in U.S. Pat. No. 5,085,629, crosslinked polysaccharide hydrogel polymers as disclosed in U.S. Pat. No. 5,057,606, and other ionically crosslinked hydrogels, as disclosed in U.S. Pat. Nos. 4,941,870, 4,286,341 and 4,878,907, the entirety of which are incorporated herein by reference.
- Crosslinked polysaccharide hydrogel polymers also are disclosed in EPA 0507604 A2.
- EPA 0645150 A-1 describes hydrogel medical devices prepared from ionically crosslinked anionic polymers (e.g., polysaccharides such as calcium alginate) or ionically crosslinked cationic polymers (e.g., chitosan, cationic guar, cationic starch, and polyethylene amine). These devices can be rapidly disintegrated in vivo upon the administration of a chemical trigger material which displaces the crosslinking ions.
- anionic polymers e.g., polysaccharides such as calcium alginate
- ionically crosslinked cationic polymers e.g., chitosan, cationic guar, cationic starch, and polyethylene amine
- Hydrogels are water-swollen polymers which offer excellent biocompatibility and have a decreased tendency to induce thrombosis, encrustation, and inflammation.
- the use of hydrogels in medical device applications has often been hindered by poor mechanical performance.
- many medical device applications for hydrogels exist where minimal stresses are encountered by the device in vivo, most applications require that the device survive high stresses during implantation.
- Inferior mechanical properties result from the swollen nature of hydrogels and the non-stress bearing nature of the swelling agent (e.g., aqueous fluids). Hydrogels suffer from low modulus, low yield stress, and low strength, when compared to non-swollen polymer systems.
- FIG. 1 is an illustration of an embodiment of a shaped medical device according to the present invention featuring alternating short segments of varying polymeric materials.
- FIG. 2 is an illustration of a stent according to one embodiment of the invention featuring alternating long segments of varying polymeric materials in the pigtail ends and the elongate tubular body of the stent.
- FIG. 3 is an illustration of a stent according to one embodiment of the present invention featuring alternating stripes of varying polymeric materials along its length.
- FIGS. 4A-4B are illustrations of stents according to embodiments of the invention featuring suture material in the form of a mesh embedded in, or coextruded with, the hydrogel.
- FIG. 4A shows suture material extruded with the hydrogel throughout the length of the stent.
- FIG. 4B shows suture material extruded with the hydrogel only within the tubular body of the stent.
- the present invention provides a means of boosting the mechanical performance of shaped medical devices comprising hydrogels, so that they may be more easily inserted into the body.
- the invention provides a means to soften such devices in vivo while retaining the structural integrity of the device, allowing the device to remain implanted within the body with minimal patient discomfort.
- the invention provides a shaped medical device having increased mechanical strength and comprising both ionic and covalent crosslinks.
- the shaped medical device according to the invention comprises a shape memory, allowing the device to be reversibly shaped in different conformations for ease of implantation and removal from the body.
- the shaped medical device has sufficient mechanical strength to permit the flow of fluids (e.g., blood) through the device.
- the shaped medical device is conformable to the shape of a stent, catheter, cannula, plug, filler, constrictor, bone anchor, plate, rod, seed, sheet or tube.
- a shaped medical device in the form of a sheet is provided as a coating for another medical device.
- the underlying medical device may comprise a dissolvable polymer, a non-dissolvable polymer, or combination non-dissolvable and dissolvable polymers.
- the shaped medical device may comprise additives to enhance the desired properties of the device.
- the shaped medical device comprises radiopaque fillers to allow visualization of the device within the body, both during and after placement at a desired target site. Additional fillers to increase the mechanical strength of the device may also be provided, including pieces of non-hydrogel material, such as suture material, or other non-dissolvable materials.
- the shaped medical device comprises an additive for medical treatment selected from the group consisting of an antiseptic, an antibiotic, an anticoagulant, a contraceptive, a nucleic acid molecule, a protein, and a medicine.
- the shaped medical device is seeded with cells.
- the shaped medical device comprising additives is provided in the form of a sheet which may be applied to a localized target site within or on the body (e.g., as a patch or a dressing) to achieve a biological effect at that target site.
- the sheet is provided as a coating to another medical device which is implanted at a target site.
- Crosslinks may be distributed homogeneously or heterogeneously throughout polymer that forms the shaped medical device.
- a shaped medical device comprises a polymer having a heterogeneous distribution of non-ionic and ionic crosslinks and a variable dissolution or degradation rate along its length.
- the shaped medical device comprises alternating segments of ionically crosslinked and non-ionically crosslinked polymer. The length of a segment may be varied to adapt the shaped medical device to a pre-selected dissolution and/or degradation rate.
- segments of non-dissolvable polymer are included.
- the non-dissolvable polymer may be in the form of a string or a mesh.
- the invention provides a shaped medical device in the form of a sheet which covers another medical device.
- the sheet-shaped medical device has a different dissolution rate from the underlying medical device.
- the sheet-shaped medical device comprises an additive (e.g., a medicine or drug) and dissolves more quickly than the underlying medical device, allowing drug release to occur while maintaining the structural integrity of the underlying device.
- the shaped medical devices according to the present invention retain their shape and stiffness during insertion into the body (e.g., by delivery through an endoscope) and can swell and soften inside the body to enhance patient comfort.
- the shaped medical device may be strengthened prior to removal from the body.
- This invention relates to shaped medical devices comprising polymer hydrogels having improved mechanical properties. More particularly, the invention relates to devices which can function as temporary implants which do not require additional surgical procedures for removal.
- the teachings of the present invention may be adapted for a variety of shaped medical devices which may be used for insertion and/or implantation into the body, including, but not limited to, biliary, urinary or vascular stents, catheters, cannulas, or components thereof, plugs or fillers, coatings, constrictors, bone anchors (e.g., screws), bone grafts (e.g., plates and rods), seeds or capsules, patches, or dressings, skin, and matrices for tissue engineering (e.g., sheets, tubes, plugs, and other macroscopic shapes).
- the shaped medical devices are optionally seeded with differentiated cells or stem cells.
- the medical devices according to the invention are suitable for both human and animal use.
- the invention is particularly applicable to shaped medical devices of tubular configuration which come in contact with one or more body fluids, such as blood, urine, gastrointestinal fluids, and bile.
- the shaped medical devices are particularly applicable for use in gastrointestinal, urogenital, cardiovascular, lymphatic, otorhinolaryngological, optical, neurological, integument and muscular body systems.
- the shaped medical devices are used in the treatment of cardiovascular, neurological, kidney, or liver disease.
- the shaped medical devices of this invention are fabricated from crosslinkable polymers which may be anionic or cationic in nature and include, but are not limited to, carboxylic, sulfate, hydroxy and amine-functionalized polymers, normally referred to as hydrogels after being crosslinked.
- crosslinkable polymers which may be anionic or cationic in nature and include, but are not limited to, carboxylic, sulfate, hydroxy and amine-functionalized polymers, normally referred to as hydrogels after being crosslinked.
- hydrogel as defined herein is a crosslinked, water-insoluble, water-containing (e.g., hydrophilic) polymeric material.
- Suitable crosslinkable polymers which may be used in the present invention include, but are not limited to, one or a mixture of polymers selected from the group consisting of polyhydroxy ethyl methacrylate, polyvinyl alcohol, polyacrylamide, poly (N-vinyl pyrolidone), polyethylene oxide, hydrolysed polyacrylonitrile, polyacrylic acid, polymethacrylic acid, polyethylene amine, polysaccharides (e.g., alginic acid, pectinic acid, carboxy methyl cellulose, hyaluronic acid, heparin, heparin sulfate, chitosan, carboxymethyl chitosan, chitin, pullulan, gellan, xanthan, carboxymethyl starch, carboxymethyl dextran, chondroitin sulfate, cationic guar, cationic starch, as well as salts and esters thereof). Polymers listed above which are not ionically crosslinkable are used in blends
- the most preferred polymers include one or a mixture of alginic acid, pectinic acid, carboxymethyl cellulose, hyaluronic acid, chitosan, polyvinyl alcohol and salts and esters thereof.
- Preferred anionic polymers are alginic or pectinic acid; preferred cationic polymers include chitosan, cationic guar, cationic starch, and polyethylene amine.
- Preferred blends comprise alginic acid and polyvinyl alcohol.
- Radiopaque fillers or other filler material can be blended into the crosslinkable polymer to enhance the radiopacity or other properties of the shaped medical device.
- Suitable radiopaque fillers include, but are not limited to, bismuth sub-carbonate, barium sulfate, bismuth oxychloride, tungsten, bismuth trioxide, tantalum, and the like.
- additives may be incorporated into the crosslinkable polymer including, but not limited to, additives for medical treatment, such as antiseptics, antibiotics, anticoagulants, contraceptives, nucleic acids [e.g., DNA (including genes, cDNAs and vectors), RNA, antisense molecules, ribozymes, PNA molecules], proteins (e.g., ligands, receptors, growth factors, cytokines, vascularizing agents, anti-vascularizing agents, antibodies, and the like), or medicines.
- additives may be added to only a portion of a medical device.
- the shaped medical device may be provided in the form of a sheet containing an additive which is used to coat another medical device which does not contain an additive.
- the other medical device may comprise a dissolvable polymer, a nondissolvable polymer, or a combination of both types of polymers. Additives for mechanical property adjustment may also be provided. In one embodiment of the invention, suture materials or other non-dissolvable materials, may be incorporated into a least a segment of the crosslinkable polymer to provide additional mechanical strength in that segment of the polymer.
- crosslinkable polymers according to the present invention may be crosslinked using either non-ionic (e.g., covalent) or ionic crosslinking.
- Ions used to ionically crosslink the polymers are polyions and may be anions or cations depending on whether the polymer is cationically or anionically crosslinkable.
- Appropriate crosslinking cations include, but are not limited to, alkaline earth metals, such as calcium, magnesium, barium, strontium, and beryllium ions; transition metals, such as iron, manganese, copper, cobalt, zinc, and silver ions; other metallic elements, such as boron, aluminum, lead, and bismuth ions; and polyamonium ions, such as + H 3 N(—CH 2 ) n —NH 3 + or + H 3 N—(CH 2 ) n —CH((CH 2 ) m —NH 3 + )((CH 2 ) p —NH 3 + ) where n is an integer ranging from 1 to 8, and m and p are integers ranging from 0 to 8 ions.
- alkaline earth metals such as calcium, magnesium, barium, strontium, and beryllium ions
- transition metals such as iron, manganese, copper, cobalt, zinc, and silver ions
- other metallic elements
- Anions are derived from polybasic organic or inorganic acids.
- Appropriate crosslinking anions include, but not limited to, phosphate, sulfate, citrate, borate, succinate, maleate, adipate and oxalate ions.
- Preferred crosslinking cations are calcium, iron, and barium ions.
- the most preferred crosslinking cations are calcium and barium ions.
- the most preferred crosslinking anion is phosphate.
- Crosslinking may be carried out by contacting the polymers with an aqueous solution containing dissolved ions.
- the crosslinkable polymers forming the shaped medical devices of this invention are crosslinked by non-ionic crosslinking mechanisms to produce a device having a higher crosslink density and improved mechanical properties, i.e., improved stiffness, modulus, yield stress and strength.
- non-ionic crosslinking mechanisms such as high energy radiation (gamma rays) or treatment with a chemical crosslinking agent which reacts with groups present in the polymer such that covalent bonds are formed connecting different portions of the polymer or between polymer strands to form a web.
- Another non-ionic crosslinking mechanism useful with respect to some classes of hydrogel polymers is physical crosslinking. This is accomplished by crystal formation or similar association of polymer blocks such that the polymer molecules are physically tied together and prevented from complete dissolution. Non-ionic crosslinking may be carried out prior to, subsequent to, or concurrently with, ionic crosslinking.
- the most preferred method for non-ionic crosslinking is contact of an ionically crosslinkable polymer with a chemical crosslinking agent because the degree of crosslinking can be more readily controlled, mainly as a function of the concentration of the crosslinking agent in the reaction medium.
- Suitable crosslinking agents are polyfunctional compounds preferably having at least two functional groups reactive with one or more functional groups present in the polymer.
- the crosslinking agent contains one or more of carboxyl, hydroxy, epoxy, halogen, amino functional groups, or hydrogen unsaturated groups, which are capable of undergoing facile nucleophilic or condensation reactions at temperatures up to about 100° C. with groups present along the polymer backbone or in the polymer structure.
- Suitable crosslinking reagents include polycarboxylic acids or anhydrides; polyamines; epihalohydrins; diepoxides; dialdehydes; diols; carboxylic acid halides, ketenes and like compounds.
- a particularly preferred crosslinking agent is glutaraldehyde.
- crosslinkable polymers are provided which possess pendant organic acid functional groups which are covalently crosslinkable with polyfunctional crosslinking agents.
- the covalent bonds between the crosslinking agents and the hydrophilic polymers are susceptible to hydrolysis in the body, releasing water-soluble components.
- organic acid functional group includes any functional group which contains an acidic, ionizable hydrogen. Examples of such functional groups include free carboxylic, free sulfuric, and free phosphoric acid groups, their metal salts and combinations thereof.
- Such metal salts include, for example, (1) alkali metal salts, such as lithium, sodium and potassium salts, (2) alkaline earth metal salts, such as calcium or magnesium salts, and (3) quaternary amine salts of such acid groups, particularly quaternary ammonium salts.
- the preferred covalent crosslinking agents are ones that can form relatively weak covalent crosslinking bonds, so that these bonds can be “unzipped” or “de-crosslinked” within the body after a desired length of time.
- polymers comprising covalent bonds that are easily hydrolysable at temperature and pH conditions inside the body are desirable.
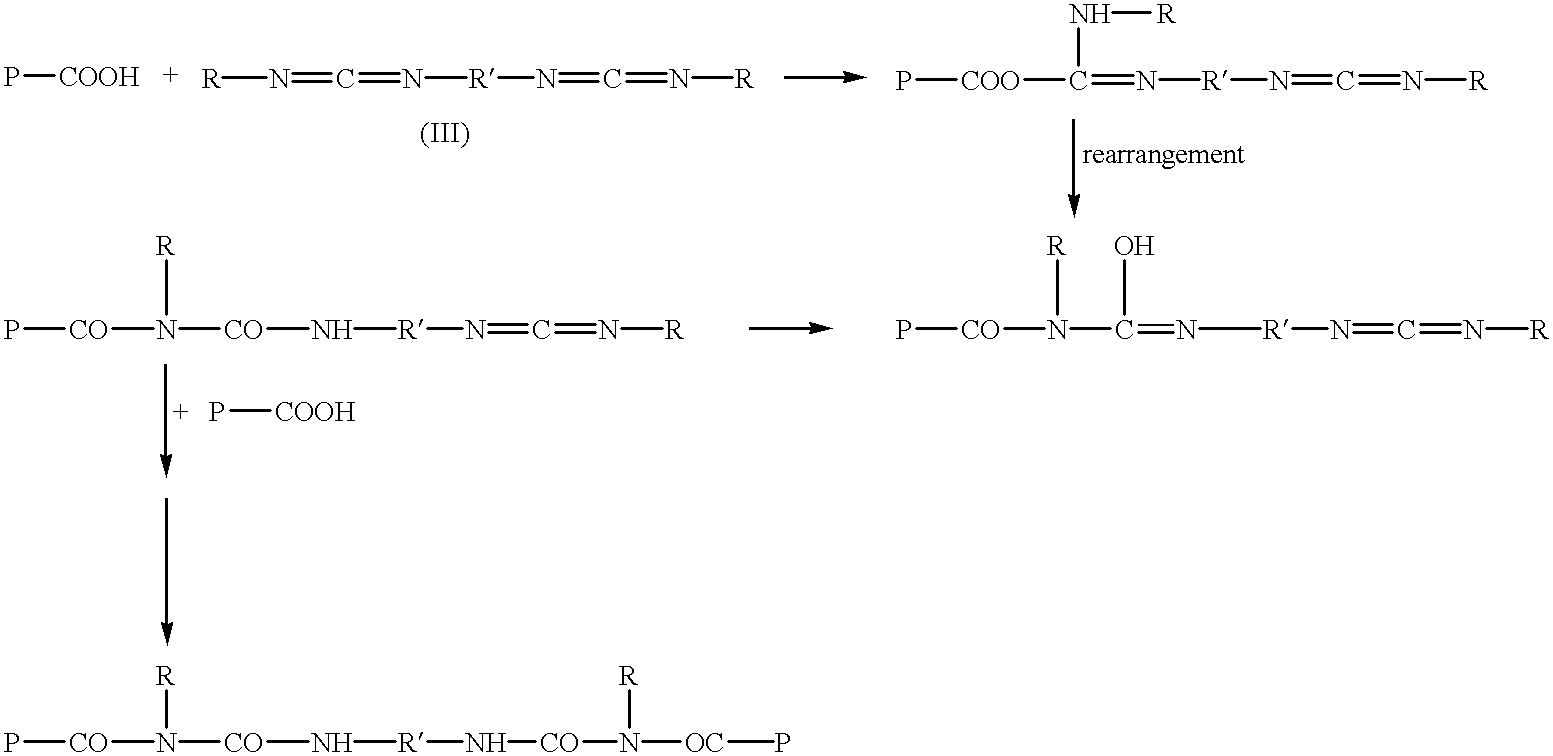
- preferred polyfunctional covalent crosslinking agents include polyfunctional aziridines, polyfunctional carbodiimides, polyisocyanate, glutaraldehyde or other polyfunctional crosslinkers wherein the functional groups are capable of reacting with the organic acid groups, or any activated forms thereof.
- polyfunctional carbodiimide as used herein includes compounds having more than one carbodiimide group corresponding to the general formula (I):
- n is an integer superior or equal to 2, and preferably ranging from 2 to 8, and wherein R and R′ are the same or different and are linear, branched, or cyclic alkyl, substituted alkyl, alkenyl, aryl, aralkyl, alkylaryl, or substituted aryl groups containing 1-18 carbon atoms wherein 1 to 3 carbon atoms may be substituted by an oxygen, an acetate, or sulfur atom or group.
- carbodiimides which are useful in this invention are those in which R and R′ are methyl, ethyl, propyl, butyl, amyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, cyclopentyl, cyclohexyl, fluoroalkyl, chloroalkyl, or bromoalkyl groups; allyl, methylallyl, aralkyl such as benzyl, phenyl ethyl, methyl benzyl, trimethyl benzyl, trityl, or the corresponding alkyl substituted naphthyl groups in which said alkyl groups contain an aggregate of from 1 to 18 carbon atoms; alkylaryl groups such as toluyl, xylyl, methyl naphthyl, or other alkyl substituted phenyl and naphthyl groups in which the alkyl
- the preferred carbodiimides for the purpose of this invention are polyfunctional polycarbodiimides derived from the reaction of mono-, di-, and tri-cycloaliphatic or saturated aliphatic isocyanates, wherein the cycloaliphatic moieties contain from 5 to about 7 carbons, and can be substituted with alkyl having 1 to about 6 carbons, and oxygen and the saturated aliphatic moieties contain from 1 to about 18 carbons.
- the reactivity of the carbodiimides with the organic acids in the crosslinkable polymer is dependent upon the nature of R and R′ in formula (I).
- R and R′ are alkyl groups.
- the most effective carbodiimides are those in which R and R′ are aryl.
- polyfunctional aziridine crosslinking agent as defined herein means a crosslinking agent with at least two nitrogen atoms. These crosslinking agents have one of the nitrogens in an aziridine ring, while the other is present in a side chain bonded to the aziridine nitrogen.
- An example of the simplest polyfunctional aziridine crosslinking agents is N-(aminoethyl) aziridine. Those polyfunctional aziridines having about three to about five nitrogen atoms per molecule of crosslinking agent are preferable.
- crosslinking agents examples include, but are not limited to, N-aminoethyl-N-aziridylethylamine, N,N-bis-2-aminopropyl-N-aziridylethylamine, and N-3,6,9-triazanonylaziridine.
- crosslinking agents include, but are not limited to, commercially available preparations of the trifunctional aziridine sold by Zeneca Resins under the trade name NeoCryl CX 100 agent having equivalent weight of 156 atomic mass units, and those preparations sold by EIT Industries under the trade name XAMA-7.
- a commercially available polyfunctional carbodiimide which is also useful in the present invention is Ucarlink XL-29SE, sold by Union Carbide.
- the covalent crosslinking agent has more than two functional groups per molecule.
- the present invention also encompasses covalent crosslinking using a combination of different polyfunctional crosslinking agents.
- polyfunctional aziridines useful in the present invention are the trifunctional aziridines of the following formula (II):
- R1 is a linear, branched, or cyclic alkyl, substituted alkyl, alkenyl, aryl, aralkyl, alkylaryl, and substituted aryl groups containing 1-18 carbon atoms wherein 1 to 3 carbon atoms may be substituted by an oxygen, an acetate, or a sulfur atom or group.
- aziridines which are useful in this invention are those in which R1 is methyl, ethyl, propyl, butyl, amyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, cyclopentyl, cyclohexyl, fluoroalkyl, chloroalkyl, or bromoalkyl group; allyl, methylallyl, aralkyl such as benzyl, phenyl ethyl, methyl benzyl, trimethyl benzyl, trityl, or the corresponding alkyl substituted naphthyl in which said alkyl groups contain an aggregate of from 1 to 18 carbon atoms; alkylaryl such as toluyl xylyl, methyl naphthyl, or other alkyl substituted phenyl and naphthyl groups in which the alkyl groups contains 1 to 18
- the concentration of the covalent crosslinking agent in the shaped medical device in this embodiment is in the range from about 0.2% to about 40% by weight solids content. In another embodiment of the invention, the concentration of covalent crosslinking agent is from about 0.5% to about 20% by weight solids content of the shaped medical device.
- the crosslinkable polymer is also combined with short segments of non-dissolvable, non-disintegratable polymer(s), or biodegradable suture material to increase the versatility of the shaped medical device.
- the segments may be spaced at regular or irregular intervals.
- Such non-dissolvable polymer(s) or biodegradable suture material(s) provide added strength to a device undergoing dissolution and/or disintegration.
- Suitable non-dissolvable polymers include, but are not limited to, silicones, and the like.
- Suitable biodegradable suture material are polymers of glycolic acid, ⁇ -caprolactone, lactic acid, or copolymers thereof, and the like.
- a shaped medical device which comprises a relatively straight section (e.g., the tubular body of a stent) which is reinforced with a biodegradable suture or braid, and a curved portion (e.g., the pigtail end of a stent) which is not reinforced.
- the curved portion anchors the shaped medical device at a particular location within the body.
- the non-reinforced curved portion of the device weakens or degrades first. This allows the relatively straight section of the device to migrate to a point of egress (e.g., in the case of a ureteral stent, to the bladder).
- the shaped medical device according to the present invention includes a weak point, or break point, in a relatively straight section of the device proximal to a curved section of the device.
- proximal means near enough to the curved section such that when the device is broken at the break point, less than 50% of the relatively straight section remains associated with the curved section.
- the break point is adjacent to the curved section.
- the shaped medical device is a stent having a relatively straight tubular body and at least one pigtail end having a weak point or break point proximal to a pigtail end.
- breaking the stent at the break point allows for migration of most of the stent into the bladder, leaving a small section in the kidney to dissolve or migrate at a later time.
- the pigtail end is not required to dissolve for the majority of the stent to migrate.
- Displacement of crosslinking ions from the shaped medical device can be accomplished by flowing a solution containing a stripping agent around and/or through the medical device in vivo.
- the stripping agent serves to displace, sequester, or bind, the crosslinking ions present in the ionically crosslinked polymer, thereby removing the ionic crosslinks.
- Stripping agents according to the present invention are polyions capable of forming stable ionic bonds with the cations or anions disclosed above. The choice of any particular stripping agent will depend on whether the ion to be displaced is an anion or a cation.
- the stripping agent will be a polyanion, while if the crosslinking agent is an anion, the stripping agent will be a polycation.
- Suitable stripping agents include, but are not limited to, organic acids and their salts or esters, phosphoric acid and salts or esters thereof, sulfate salts and alkali metal or ammonium salts.
- stripping agents include, but are not limited to, ethylene diamine tetraacetic acid, ethylene diamine tetraacetate, citric acid and its salts, organic phosphates, such as cellulose phosphate, inorganic phosphates, such as, pentasodium tripolyphosphate, mono and dibasic potassium phosphate, sodium pyrophosphate, phosphoric acid, trisodium carboxymethyloxysuccinate, nitrilotriacetic acid, maleic acid, oxalate, polyacrylic acid, as well as sodium, potassium, lithium, calcium and magnesium ions.
- Preferred agents are citrate, inorganic phosphates, and sodium, potassium and magnesium ions. The most preferred agents are inorganic phosphates and magnesium ions.
- Specific methods for introduction of the stripping agent include introduction through the diet of the patient or through parenteral feeding, introduction of a solution directly onto the shaped medical device, such as by insertion of a catheter which injects the agent within the device, or through an enema.
- the shaped medical device is an implanted calcium alginate ureteral stent which is strippable by phosphate anions.
- the device is stripped by including in the patient's diet, materials which bind phosphate (e.g., calcium salts) to lower the content of PO 4 ⁇ 3 present in the urine (normally up to about 0.1%.). Achievement of levels of phosphate in the urine of from 0.2 to 0.3% will result in the in vivo stripping of the calcium ions from the calcium alginate stent. Lower levels of phosphate in the urine will also result in a more gradual stripping of the calcium ions, but higher levels are preferred for rapid stripping of the calcium.
- materials which bind phosphate e.g., calcium salts
- the stripping process may be reversed to re-stiffen the shaped medical device which facilitates surgical removal of the device from the body. This may be accomplished by flowing a source of crosslinking ions through and/or around the shaped medical device to ionically re-crosslink the implant, essentially the reverse of the stripping process described above. Dietary modifications can also be used to re-crosslink the shaped medical device in vivo, e.g., by eliminating phosphate binders (endogenous or exogenously added) from the diet and adding foods or other dietary supplements which provide or generate phosphate ions in the body.
- the present invention also provides several means of controlling or slowing the dissolution rate of the ionically crosslinked material upon exposure to a stripping agent.
- a polymer that has a higher ionic crosslinking content will dissolve more slowly than a polymer that has a lower ionic crosslinking content.
- By modifying the amount of ionic crosslinking in different portions of the shaped medical device it is possible to design devices which dissolve in at non-uniform rates along different portions of the device.
- a stent is provided which has pigtail ends with a relatively low ionic crosslinking content compared to the tubular body of the stent. In this embodiment of the invention, the pigtail ends dissolve faster than the tubular body.
- the stent When the stent is a ureteral stent placed in the kidney, the dissolution of a pigtail end located in the kidney permits migration of the stent within the ureter and into the bladder.
- a similar result is achieved by modifying the thickness of the walls of the shaped medical device, e.g., thinner walls will dissolve faster than thicker walls.
- a stent is provided having pigtail ends with thinner walls than its tubular body.
- a medical device in the form of a sheet may be provided as a coating for an underlying medical device.
- the composition of the sheet is optimized to allow the sheet to dissolve more quickly than the underlying medical device.
- the sheet-shaped medical device comprises an additive (e.g., a medicine or drug)
- the sheet will dissolve first, delivering a desired amount of additive to a target site, while the underlying device maintains its structural integrity at the target site.
- the underlying device is a stent and is placed in a blood vessel
- the sheet portion of the device may be used to deliver an anti blood vessel occluding agent, while the stent portion of the device acts as a prosthesis to maintain the opening of the blood vessel.
- the underlying medical device may be completely non-dissolvable.
- a similar scale may be established for anionic crosslinking ions.
- the pK a values of acids and bases are well known in the art.
- the rate of dissolution of a shaped medical device may be controlled by selecting an ion that has a greater affinity (or basicity) to slow the dissolution process.
- the stent is crosslinked in the pigtail end with a low affinity (or basicity) ion (such as Mg 2+ or Ca 2+ for anionic polymers), and with a high affinity (or basicity) ion (such as Ba 2+ or Zn 2+ ) in the elongate tubular portion of the stent.
- the shaped medical device e.g., stent
- the shaped medical device may contain both types of crosslinking cations, i.e., low and high affinity, such as barium and calcium ions.
- the cations are provided in differing concentrations in different sections of the device.
- the ends of the shaped medical device e.g., the pigtail portions, in the case of a stent
- the body of the device e.g., the tubular body in the case of a stent
- high affinity cations e.g., Ba 2+
- low affinity cations e.g., Ca 2+
- low affinity cations e.g., Ca 2+
- the body of the device e.g., the tubular body in the case of a stent
- high concentrations of high affinity cations e.g., Ba 2+
- low affinity cations e.g., Ca 2+
- Ureteral stents manufactured according to the present invention substantially reduce the risk of hydronephrosis of the kidney which results from obstruction of the ureter by stent fragments.
- An added benefit of the stents according to the present invention is increased patient comfort, since the stents are made of a temporary material which will resorb, biodegrade, disintegrate, or erode, after placement in vivo, eventually becoming expelled through the urethra upon urination.
- a ureteral stent which comprises an initially high durometer (e.g., about 50-100 Shore A) for ease of placement in the body which reduces to a lower durometer (e.g., about 20-50 Shore A) for enhanced patient comfort while it functions as a stent.
- at least one end of the stent comprises a coiled end.
- the coiled end residing in the kidney continues to soften and weaken, ultimately migrating in a single intact piece through the ureter and into the bladder. Once in the bladder, the material continues to break down into smaller pieces either by chemical breakdown or physical agitation from an expanding or contracting bladder. In this part of the body, fragments are harmlessly expelled.
- a ureteral stent is produced which is composed of an alginate material and reversibly crosslinked with 19% by weight of barium and 0.17% by weight of calcium.
- the stent has a pigtail or coil on either end to prevent migration after initial placement.
- the stent also contains a radiopacifier (e.g., bismuth subcarbonate or barium sulfate) to make it visible under fluoroscopy, whether fully intact or in fragments.
- the stent has an initial durometer of about Shore 60A, but softens to about Shore 30A after a short period of time. This softer durometer provides for increased patient comfort.
- the pigtail strength maintains a minimum of 20 grams for at least 48 hours after insertion into the body.
- the pigtail's strength diminishes to a point that allows the stent to migrate to a point of egress (e.g., the bladder) prior to fragmenting. After migrating, the stent continues to break into smaller pieces or erode to a smaller diameter so that it can be easily and readily expelled.
- a point of egress e.g., the bladder
- Sodium alginate is first extruded as a hollow tube into a bath containing barium ions.
- the ends of the stent are then consecutively dipped into a bath containing a sequestant for barium ions, such as ethylene diamine tetraacetic acid or phosphate.
- the ends are then coiled to form the pigtail and dipped into a bath containing calcium ions. Crosslinking of the ends with calcium also set their coiled shape.
- dual crosslinked shaped medical devices are provided, i.e., containing both non-ionic and ionic crosslinks.
- the ionic crosslinks can be easily and selectively displaced in vivo after implantation of the device in the body while the non-ionic crosslinks are not significantly removed. Consequently, the shaped device swells and softens in vivo, enhancing patient comfort, while retaining its original non-ionically crosslinked shape configuration to a large degree.
- the covalent bonds in the dual crosslinked shaped medical devices make the device water-insoluble.
- the device will soften upon removal of the ionic crosslinking agents or hydration and will be able to migrate in the body.
- the covalent bonds are also susceptible to hydrolysis within the body and thus the devices will degrade over time.
- the dual crosslinked shaped medical devices comprise covalent and ionic crosslinks throughout the device in the following ratio ranges: (1) covalent crosslinking occupies 0.1%-100% of the available crosslinking sites of the hydrophilic polymer; and (2) ionic crosslinking occupies 0%-99.9% of all available crosslinking sites of the hydrophilic polymer.
- the crosslinking sites are the sites on the hydrophilic polymer where a pendent organic acid group is available to react with a crosslinking agent.
- the dissolution and disintegration of the shaped medical device occurs simultaneously throughout the device, but at a different rate for each type of crosslinking.
- dissolution i.e., removal of the ionic crosslinks
- disintegration i.e., hydrolysis of the covalent crosslinks.
- the shaped medical devices first soften upon the loss of ionic crosslinks while maintaining the overall shape and structural cohesion provided by the covalent crosslinks.
- Such softened shaped medical devices may provide for easy and painless removal of the device by natural migration through body vessels. In the case of a temporary ureteral stent, for example, the softened stent would migrate out of the ureter and kidney to the bladder where it would slowly disintegrate and be eliminated as water-soluble components or by-products are released.
- the crosslinked shaped medical devices according to the invention are shaped by extrusion, molding, compression of the polymers, or other means, before the polymer is covalently or ionically crosslinked.
- the shaped medical devices in final form are crosslinked to maintain their shape.
- One process for manufacturing a shaped medical device according to the present invention comprises introducing a solution comprising ionically crosslinkable polymer through a die to form a tube while simultaneously pumping a solution comprising crosslinking ion through the die.
- Linear device or pre-device configurations such as fibers, rods, tubes or ribbons can be manufactured in accordance with the present invention by using a spinning device in which an aqueous solution of an ionically crosslinkable matrix polymer is forced through a shaping die into a crosslinking bath containing the crosslinking ions.
- the product after crosslinking is typically described as a hydrogel.
- the hydrogel may be used as made, or further given a three-dimensional shape through treatment in a crosslinking solution after being forced into the desired shape. After equilibration, the hydrogel will retain the new three-dimensional shape.
- the device may be used in its hydrogel form or in a dehydrated form. During dehydration, the three-dimensional shape is retained.
- the shaped medical device is shaped by wet spinning to form a tube.
- the shaped medical device is formed into a tubular shape by molding a crosslinkable polymer in a one- or two-part reaction injection molding system.
- the term “tube” or “tubular shape” refers to a generally cylindrical device having a substantially uniform circular cross-section throughout the device and to a device having cross-sections of varying shapes and sizes, so long as the device has a hollow passageway with at least one opening through which fluid may enter and at least one opening through which fluid may leave.
- the shaped medical device may be stored wet or dry.
- the medical device is stored in an aqueous solution or buffer or (e.g. deionized water, or water containing hygroscopic agents, such as glycerol, sorbitol, sucrose, and the like).
- the shaped medical device is dried prior to storage.
- the crosslinkable polymer shaped into the desired implant is dipped consecutively into two baths, each bath containing one type of crosslinking agent.
- the entirety of the crosslinkable polymer may be dipped into a first bath containing a solution of crosslinking cations to ionically crosslink a portion of the available crosslinking sites of the polymer; then, the least partially ionically crosslinked polymer is dipped into a second bath containing a solution of covalent crosslinking agent to covalently crosslink a portion, or all of, the remaining available crosslinking sites of the hydrophilic polymer.
- each type of crosslinking may be varied by varying the length of time of exposure of the shaped medical device to each type of crosslinking agent or, alternatively, by masking portions of the device.
- the crosslinkable polymer and crosslinking agents may be cast into a sheet or first to test the mechanical properties of the crosslinked polymer.
- the same concentration of crosslinkable polymer and crosslinking agents may used to form a shaped medical device having a more complex shape, such as a tube.
- a biliary or ureteral stent is fabricated which has improved modulus (stiffness) properties due to dual crosslinking.
- modulus stiffness
- Such stents are robust enough and sufficiently resistant to buckling to allow them to be readily inserted into the appropriate part of the body with an endoscope.
- the ionic crosslinks present in the stents can be selectively, at least partially stripped, either directly by the physician, by dietary means, or by means of exposure to natural body fluids such as bile or urine.
- the modulus of the stents are lowered and the stents soften and swell in body fluids, resulting in a more comfortable and conformable element and a larger lumen through which body fluids may flow.
- An enlarged lumen is preferred in tubular shaped medical devices to allow higher flow rates, to enhance the anchoring of the device to the body, and to decrease the likelihood of occlusion during use.
- sodium alginate is extruded into a stent shape by precipitating alginate tubing into a calcium bath.
- the stent is placed on a pre-shaped fixture with pigtail ends and calcium ions are stripped by dipping into a bath containing stripping agents.
- the alginate tubing is then covalently crosslinked with a polyfunctional crosslinking agent.
- the stent may be crosslinked to various degrees along the length of the stent to optimize the function of the shaped medical device.
- the pigtails will be formed using sufficient covalent crosslinking to prevent their breaking in the kidney; however, they will also be designed to become soft after a certain time so that as they lose their strength they can be straightened and migrate out of the kidney.
- a ureteral stent is made of different polymers (e.g., either entirely hydrophilic or at least partially hydrophilic) along the length of the stent.
- the pigtail ends are made of dissolvable material and the tubular body is made of a combination of semi-dissolvable and non-dissolvable materials. If non-dissolvable material is used for any part of the stent, the material should be short and flexible enough to migrate inside the urinary track (e.g., a piece of suture material).
- a long stent can be made by joining one or more short non-dissolvable pieces to a dissolvable portion of the stent. The joining material in this embodiment should be dissolvable.
- batch 1 comprises a mixture of hydrophilic polymers with a covalent crosslinking agent
- batch 2 comprises a mixture of hydrophilic polymers with a ionic crosslinking agent
- batch 3 consists essentially of the hydrophilic polymer by itself.
- shaped medical devices having various sections made of differing types of crosslinking are prepared by molding or extruding the implant with material selected alternatively from any one of batches 1 , 2 , and 3 . The resulting extruded or molded implant may be further treated by heat, for example, to set the covalent crosslinking process, or further exposed to ionic crosslinking agents.
- dual crosslinked shaped medical devices are made of discrete segments of non-ionically and ionically crosslinked hydrogel polymer.
- different portions of the device dissolve or disintegrate at different rates, leading to the breakdown of the shaped medical device in small chunks or segments that can migrate through the body vessels without causing undesirable obstructions of these vessels.
- the segments of the implant desired to be ionically crosslinked are dipped into a bath containing an ionic crosslinking agent.
- the remaining segments desired to be covalently crosslinked are then dipped into a bath containing a covalent crosslinking agent.
- Such a process is particularly suitable for the preparation of ureteral stents.
- the pigtail ends of the stent are ionically crosslinked and the tubular body of the stent is covalently crosslinked.
- selective de-crosslinking and re-crosslinking is used to change the shape of the medical device, in vitro or in vivo. In in vivo applications, this allows the device to be placed in the body in a shape that facilitates insertion and to be retained in the body in a shape that facilitates anchoring and patient comfort.
- a secondary shape is imparted to the medical device prior to implantation in the body. This is accomplished by deforming the primary shape of a device which is crosslinked at least non-ionically, setting the device in the deformed shape by ionic crosslinking, and implanting the device in the body in the deformed shape. Stripping the ions in vivo as described above will cause the device to revert in vivo to its primary non-ionically crosslinked shape.
- an ionically crosslinkable polymer is formed into a primary shape and subjected to non-ionic crosslinking conditions to form a non-ionically crosslinked hydrogel having this primary shape.
- Non-ionic crosslinking can be carried out by the methods described above, and is preferably carried out by extruding the polymer into a bath containing a sufficient amount of one or more of the non-ionic crosslinking agents to form a shape-retaining hydrogel. Next, a secondary shape is imparted to the non-ionically crosslinked hydrogel and the hydrogel is then subjected to ionic crosslinking conditions to ionically crosslink the hydrogel while retaining this secondary shape.
- an ionically crosslinkable polymer is formed into a primary shape and subjected to both non-ionic and ionic crosslinking conditions to form a crosslinked polymer having the primary shape and containing both ionic and non-ionic crosslinks.
- an ionically and non-ionically crosslinked shaped polymer is prepared as above. Then, the shaped polymer is selectively stripped ex vivo of at least a portion or essentially all of the crosslinking ions; the shaped polymer is then conformed to a secondary shape, e.g., bent around a wire, stretched, compressed, or the like. The shaped polymer is subsequently ionically re-crosslinked while retained in the secondary shape.
- the stripping step described above can occur immediately prior to, or subsequent to the secondary shaping step, but preferably subsequent to the secondary shaping step and prior to the ionic re-crosslinking step.
- the shaped medical device is of hollow, tubular configuration, such as a stent.
- the stent is both ionically and non-ionically crosslinked, it may be selectively stripped of crosslinking ions.
- the stent is stretched to form a narrower stent which facilitates insertion into the body, ionically crosslinked or re-crosslinked in the stretched state to fix the stent in the stretched state, implanted in the body and then re-stripped in vivo of the ionic crosslinks to produce a softer shaped device having a wider lumen.
- stent shapes such as pigtail ends, flaps, curves, and the like can be developed in vivo by subjecting devices having these primary initial shapes to the process described above, i.e., deforming the primary shape ex vivo and reforming the primary shape in vivo.
- the stripping step described above is preferably accomplished by dipping or spraying the crosslinked shaped device with an aqueous electrolyte solution for an appropriate time to selectively strip the crosslinking ions from the device.
- Preferred electrolytes for ex vivo stripping are chlorides of monovalent cations such as sodium, potassium or lithium chloride, as well as other stripping salts described above.
- the concentration of the electrolyte salt in the solution may range from about 1 weight percent (wt %) up to the solubility limit.
- the solution may also contain plasticizing ingredients, such as glycerol or sorbitol, to facilitate inter- and intra-polymer chain motion during, and after, secondary shaping. Secondary shaping of the medical device may be done by hand, i.e., using pinning boards or jig pins, or by using shaped presses or molds.
- the device may be ionically crosslinked or re-crosslinked in the secondary shape by contacting the device, while retaining the secondary shape, with an aqueous solution containing the crosslinking ions described above. After crosslinking, the device will essentially retain the secondary shape.
- the ionically crosslinked, shaped polymer prepared as above is then subjected to non-ionic crosslinking, e.g., high energy radiation or by contact under appropriate acidic or basic conditions with the appropriate chemical crosslinking agent.
- Crosslinking is preferably carried out by soaking the polymer in an aqueous solution containing a water soluble crosslinking agent such as glutaraldehyde, ethylene diamine or a lower alkylene glycol.
- a water soluble crosslinking agent such as glutaraldehyde, ethylene diamine or a lower alkylene glycol.
- concentration of crosslinking agent in solution may range from about 0.25 to about 10 wt %, more preferably from about 0.5 to 5.0 wt %.
- the degree of non-ionic crosslinking is controlled as a function of the concentration of the crosslinking agent in solution.
- the level should be selected such that a stiffer, higher modulus shaped medical device is produced which will revert to a soft, stretchy, shape retaining device after removal of the ionic crosslinks.
- the crosslinking process may also be conducted by first crosslinking the polymer non-ionically, followed by ionic crosslinking, essentially the reverse of the process described above.
- the ionically crosslinkable polymer composition includes polymers which are partially water soluble
- additives such as borax, boric acid, alkali metal salts and/or a lower alcohol such as methanol.
- the various steps may be performed at any suitable temperature, e.g., at room temperature or at temperatures up to about 100° C. Preferably, soaking steps are conducted at room temperature. Moreover, the steps may be performed one immediately after another, or a drying step (e.g., air-drying) may be interposed between one or more steps. Additionally, the shaped medical device may be sterilized after the sequence of secondary-shaping steps.
- hydrogel systems which may be prepared in accordance with this invention are prepared by the following procedures:
- a solution of sodium alginate is extruded through a tube die into a calcium chloride bath while calcium chloride solution is simultaneously introduced through the lumen of the tube.
- This ionically crosslinked tube is then covalently crosslinked by treatment with an aqueous solution containing glutaraldehyde.
- the now covalently and ionically crosslinked gel has a higher crosslink density and therefore higher modulus than a similar tube having only the covalent or only the ionic crosslinks.
- the tube therefore has higher stiffness and improved resistance to buckling than a tube having the covalent or ionic crosslinks alone.
- Suitable ions which will displace the calcium crosslinking ions include phosphate, sulfate, carbonate, potassium, sodium and ammonium.
- the device When implanted the device may be stiffened and strengthened during removal from the body via exposure of the device to an infusion fluid which contains a solution of the crosslinking ions (e.g., calcium).
- a solution of sodium alginate may be contacted with calcium chloride and cast as a film into a container (e.g., a culture dish or petrie plate), thereby forming a shaped medical device in the form of a sheet.
- a container e.g., a culture dish or petrie plate
- the sheet which may be rolled around another tubular shaped medical device, such as the one disclosed in Example 1, e.g., to form a coating for a stent.
- Additives e.g., medicines or drugs
- a blend of polyvinyl alcohol (PVA) and sodium alginate may be dispersed or dissolved in water, extruded into a bath containing calcium ions.
- the bath additionally contains non-solvent conditions for the polyvinyl alcohol.
- the polyvinyl alcohol component of the formed article is then covalently crosslinked with an aqueous solution containing glutaraldehyde.
- the shaped medical device is now ready for insertion and/or implantation. After implantation, the article may be softened and swollen by removal of the ionic crosslinks as above. Removal of the ionic crosslinks may also optionally allow the alginate to fully, or partially dissolve, in the body fluids, leaving behind a less dense, more porous hydrogel.
- the morphology of the final hydrogel device may be controlled by optimizing of polyvinyl alcohol molecular weight, degree of crosslinking, solvent composition, the molecular weight of the alginate used, the state of the alginate salt (dissolved, particulated, gel), the alginate monomer makeup, as well as the temperature, pressure, mix time, solution age, and rheological factors during manufacture.
- the blend of PVA and sodium alginate described in (b) above may be used to make a stent having a shape memory feature to gain increased lumen size after deployment in vivo.
- a tube is made by extruding the mixture through a tube die into a concentrated calcium chloride bath, optionally containing other salts and boric acid. The tube is then transferred into a bath which contains calcium chloride and a chemical crosslinker (e.g., glutaraldehyde). After allowing the reaction to proceed, the tube will become a covalently crosslinked PVA/calcium alginate system. The tube is immersed in concentrated potassium chloride solution to remove the calcium crosslinks from the alginate while preventing the alginate from dissolving.
- a chemical crosslinker e.g., glutaraldehyde
- the tube is then stretched to form a longer length tube having a more narrow lumen. While in this stretched configuration, the tube is immersed into concentrated calcium chloride solution to re-crosslink the alginate. The tube is frozen into the longer length, narrow lumen configuration. Upon insertion into the body, the tube will return to its original shorter length, large lumen configuration, as the calcium is stripped from the alginate. The alginate will eventually dissolve, leaving behind a more porous glutaraldehyde crosslinked PVA tube. Other imposed shapes may be used to accommodate insertion into the body in a compact form, followed by shape change upon displacement of the ionic crosslinks.
- Propyleneglycol alginate is covalently crosslinked with ethylene diamine under basic conditions and ionically crosslinked with calcium ions.
- This covalently and ionically crosslinked material will exhibit higher stiffness than the material crosslinked with covalent linkages only. Removal of the ionic crosslinks will occur in vivo after deployment in body fluid.
- a stent, catheter, or cannula can be manufactured from this material, implanted while both ionically and covalently crosslinked, the device softens in vivo as the ionic crosslinks are displaced.
- a shaped device of this construction provides stiffness for implantation and softness for patient comfort.
- the deionized water was weighed into a 4 oz. jar, while stirring the water, the PVA and sodium alginate were added and mixed until uniform.
- the jar was capped and heated to 100° C. to dissolve the ingredients.
- the jar was cooled to 37° C., then the bismuth subcarbonate (radiopaque filler) which had been sifted through a 325 mesh screen was added and the composition was mixed with a jiffy mixer until uniform.
- the samples were loaded into 30 cc syringes, centrifuged to remove air, then extruded through a tubing die into a coagulant solution.
- the coagulant solution was made from 100 grams of calcium chloride dehydrate, 30 grams of sodium chloride, 50 grams of boric acid and 820 grams of deionized water.
- the spun tubing was left in the coagulant solution overnight. Lengths of tubing were then soaked in a glutaraldehyde/coagulant solution mixture to covalently crosslink the sample. Glutaraldehyde levels were tested from 0.5% by weight to 12.5% by weight.
- the pH was adjusted to 1.5 using 20% HCl solution. After reacting overnight at room temperature, the tubes were examined and then immersed in 0.4% sodium phosphate solution to strip the ionic crosslinks. Results are shown in Table 2.
- Gutaraldehyde (wt %) 0.5% 1.0% 5.0% 12.5% 15/5 (PVA/Alginate soft, stretchy slightly stiffer, but stiff, brittle wt. ratio) stiffer still soft 15/7.5 soft, stretchy slightly much stiffer stiff, brittle (PVA/Alginate stiffer wt. ratio) 20/5 (PVA/Alginate soft, stretchy slightly stiff, brittle stiff, brittle wt. ratio) stiffer
- a shaped medical device in the form of a ureteral stent 10 having a pigtail end 16 for positioning into the kidney of a patient, a tubular elongate body 12 for positioning into the ureter, and a second pigtail end 17 for positioning into the bladder.
- the stent 10 optionally comprises drainage holes 14 , throughout the elongate body 12 and the pigtail ends 16 and 17 .
- the stent 10 is molded or extruded from at least two different materials arranged in segments 18 and 20 .
- Each segment may comprise: (1) an entirely ionically crosslinked hydrogel; (2) an entirely non-ionically crosslinked hydrogel; (3) a mixture of non-ionically and ionically crosslinked hydrogels; or (4) non-dissolvable polymer.
- segment 18 is a non-dissolvable polymer while segment 20 comprises at least ionic crosslinks.
- segments 18 and 20 are alternatively distributed along the length of the stent body at regular intervals. The length of segment 18 is short relative to the length of segment 20 to facilitate removal of the non-dissolvable portion of the stent from the body.
- Stent Comprising Pigtail End Segments and a Tubular Body Segment.
- FIG. 2 shows a uretral stent 10 having only three segments, a tubular body segment 20 flanked by two pigtail end segments 18 .
- Segments 18 in the pigtail ends 16 and 17 represent a material having a faster rate of dissolution, while segment 20 represents a material having a slower rate of dissolution.
- FIG. 3 shows a ureteral stent 10 according to one embodiment of the invention comprising segments 18 and 20 which are in the form of stripes or strings that run parallel to the longitudinal axis of the stent 10 from one pigtail end 16 to the other 17 .
- the thickness of each stripe or string may be varied to achieve the desired strength and desired disintegration rate of the stent 10 .
- the strings themselves comprise suture material or some other non-dissolvable material.
- FIG. 4A shows a ureteral stent according to the invention comprising segments 22 which form a mesh of non-dissolvable material or suture material that is co-extruded with the hydrogel and stretches along the length of the stent from one pigtail end 16 to the other 17 .
- FIG. 4B represents an alternative embodiment of the stent where the mesh of non-dissolvable material 22 or suture material is co-extruded with the hydrogel 18 only in the elongate tubular body portion 12 .
Abstract
Description
| TABLE 1 | ||||||||
| PVA/alginate (wt. rat.) | 15/5 | 20/5 | 15/7.5 | 20/5 | ||||
| Deionized water | 72 | g | 67.5 | g | 69.7 | g | 74.25 | g |
| PVA | 13.5 | g | 18.0 | g | 13.5 | g | 19.8 | g |
| Sodium alginate | 4.5 | g | 4.5 | g | 6.75 | g | 4.95 | g |
| Bismuth subcarbonate | 9.68 | g | 9.77 | g | 9.69 | g | 9.9 | g |
| TABLE 2 | ||||
| Gutaraldehyde | ||||
| (wt %) | 0.5% | 1.0% | 5.0% | 12.5% |
| 15/5 (PVA/Alginate | soft, stretchy | slightly | stiffer, but | stiff, brittle |
| wt. ratio) | stiffer | still soft | ||
| 15/7.5 | soft, stretchy | slightly | much stiffer | stiff, brittle |
| (PVA/Alginate | stiffer | |||
| wt. ratio) | ||||
| 20/5 (PVA/Alginate | soft, stretchy | slightly | stiff, brittle | stiff, brittle |
| wt. ratio) | stiffer | |||
Claims (38)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/512,698 US6368356B1 (en) | 1996-07-11 | 2000-02-25 | Medical devices comprising hydrogel polymers having improved mechanical properties |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/679,609 US6060534A (en) | 1996-07-11 | 1996-07-11 | Medical devices comprising ionically and non-ionically crosslinked polymer hydrogels having improved mechanical properties |
| US12225699P | 1999-02-25 | 1999-02-25 | |
| US12217699P | 1999-02-25 | 1999-02-25 | |
| US09/496,709 US6184266B1 (en) | 1996-07-11 | 2000-02-02 | Medical devices comprising cross-linked hydrogels having improved mechanical properties |
| US09/512,698 US6368356B1 (en) | 1996-07-11 | 2000-02-25 | Medical devices comprising hydrogel polymers having improved mechanical properties |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/496,709 Continuation-In-Part US6184266B1 (en) | 1996-07-11 | 2000-02-02 | Medical devices comprising cross-linked hydrogels having improved mechanical properties |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US6368356B1 true US6368356B1 (en) | 2002-04-09 |
Family
ID=27494415
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/512,698 Expired - Lifetime US6368356B1 (en) | 1996-07-11 | 2000-02-25 | Medical devices comprising hydrogel polymers having improved mechanical properties |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US6368356B1 (en) |
Cited By (102)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020133054A1 (en) * | 2001-02-28 | 2002-09-19 | Gregory Murphy | Sizing apparatus and method for use during ventricular restoration |
| US6468312B1 (en) * | 1998-07-26 | 2002-10-22 | Klaus Rennebeck | Ureapoietic organ replacement |
| US20030021762A1 (en) * | 2001-06-26 | 2003-01-30 | Luthra Ajay K. | Polysaccharide biomaterials and methods of use thereof |
| US20030068817A1 (en) * | 2001-04-30 | 2003-04-10 | Dan Gazit | Vascular tissue engineering |
| US20030083433A1 (en) * | 2001-10-30 | 2003-05-01 | James Susan P. | Outer layer having entanglement of hydrophobic polymer host and hydrophilic polymer guest |
| US20030148995A1 (en) * | 2000-07-19 | 2003-08-07 | Estelle Piron | Polysaccharide crosslinking, hydrogel preparation, resulting polysaccharide(s) and hydrogel(s), uses thereof |
| US20030181940A1 (en) * | 2001-02-28 | 2003-09-25 | Gregory Murphy | Ventricular restoration shaping apparatus and method of use |
| US20030204238A1 (en) * | 2002-04-26 | 2003-10-30 | Eugene Tedeschi | Coated stent with crimpable coating |
| US6673453B2 (en) * | 2001-06-12 | 2004-01-06 | Biocoat Incorporated | Coatings appropriate for medical devices |
| US20040059102A1 (en) * | 2002-09-23 | 2004-03-25 | Lucent Technologies Inc. | Compositions that reversibly gel and de-gel |
| US20040062808A1 (en) * | 2002-09-23 | 2004-04-01 | Lucent Technologies Inc. | Agent delivery system capable of selectively releasing an agent |
| US20040102558A1 (en) * | 2002-11-25 | 2004-05-27 | Tung-Liang Lin | Polymeric coatings containing a pH buffer agent |
| WO2004026360A3 (en) * | 2002-09-19 | 2004-07-22 | Medtronic Inc | A medical assembly suitable for long-term implantation and method for fabricating the same |
| US20040151752A1 (en) * | 1999-04-12 | 2004-08-05 | Chee-Youb Won | Hydrogel entrapping therapeutic agent and stent with coating comprising this |
| US20040249408A1 (en) * | 2001-09-05 | 2004-12-09 | Chase Medical, Lp | Method and device for endoscopic surgical ventricular repair |
| US20050008626A1 (en) * | 2001-12-07 | 2005-01-13 | Fraser John K. | Methods of using adipose tissue-derived cells in the treatment of cardiovascular conditions |
| US20050020506A1 (en) * | 2003-07-25 | 2005-01-27 | Drapeau Susan J. | Crosslinked compositions comprising collagen and demineralized bone matrix, methods of making and methods of use |
| US20050048034A1 (en) * | 2001-12-07 | 2005-03-03 | Fraser John K. | Methods of using regenerative cells to promote wound healing |
| US20050048036A1 (en) * | 2001-12-07 | 2005-03-03 | Hedrick Marc H. | Methods of using regenerative cells in the treatment of inherited and acquired disorders of the bone, bone marrow, liver, and other tissues |
| US20050048033A1 (en) * | 2001-12-07 | 2005-03-03 | Fraser John K. | Methods of using regenerative cells in the treatment of renal diseases and disorders |
| US20050048644A1 (en) * | 2001-12-07 | 2005-03-03 | Hedrick Marc H. | Methods of using regenerative cells in the treatment of musculoskeletal disorders |
| US20050058632A1 (en) * | 2001-12-07 | 2005-03-17 | Hedrick Marc H. | Cell carrier and cell carrier containment devices containing regenerative cells |
| US20050084961A1 (en) * | 2001-12-07 | 2005-04-21 | Hedrick Marc H. | Systems and methods for separating and concentrating regenerative cells from tissue |
| US20050095228A1 (en) * | 2001-12-07 | 2005-05-05 | Fraser John K. | Methods of using regenerative cells in the treatment of peripheral vascular disease and related disorders |
| US20050158359A1 (en) * | 2003-05-06 | 2005-07-21 | Epstein Samuel J. | Processes for producing polymer coatings for release of therapeutic agent |
| US20050240278A1 (en) * | 2004-04-26 | 2005-10-27 | Peter Aliski | Stent improvements |
| US20050240280A1 (en) * | 2004-04-26 | 2005-10-27 | Peter Aliski | Ureteral stent |
| US20050240277A1 (en) * | 2004-04-26 | 2005-10-27 | Peter Aliski | Stent with flexible elements |
| US20050260174A1 (en) * | 2001-12-07 | 2005-11-24 | Fraser John K | Systems and methods for treating patients with processed lipoaspirate cells |
| US20050260175A1 (en) * | 2001-12-07 | 2005-11-24 | Hedrick Marc H | Systems and methods for isolating and using clinically safe adipose derived regenerative cells |
| WO2006069349A2 (en) * | 2004-12-22 | 2006-06-29 | Cytori Therapeutics, Inc. | Cell-loaded prostheses for regenerative intraluminal applications |
| US20060204556A1 (en) * | 2001-12-07 | 2006-09-14 | Cytori Therapeutics, Inc. | Cell-loaded prostheses for regenerative intraluminal applications |
| WO2007042281A2 (en) | 2005-10-12 | 2007-04-19 | Thomas Freier | Processing of chitosan and chitosan derivatives |
| US20070254041A1 (en) * | 2006-05-01 | 2007-11-01 | Drapeau Susan J | Demineralized bone matrix devices |
| US20080065192A1 (en) * | 2006-09-13 | 2008-03-13 | Medtronic Vascular, Inc. | Compliance Graded Stent |
| US20080071384A1 (en) * | 2006-09-19 | 2008-03-20 | Travis Deal | Ureteral stent having variable hardness |
| WO2008036711A1 (en) * | 2006-09-22 | 2008-03-27 | Boston Scientific Scimed, Inc. | Stent with soluble bladder retention member |
| US20080097349A1 (en) * | 2006-06-16 | 2008-04-24 | Boston Scientific Scimed, Inc. | Partially soluble implantable or insertable medical devices |
| US20080140451A1 (en) * | 2005-01-10 | 2008-06-12 | Cytori Therapeutics, Inc. | Devices and Methods for Monitoring, Managing, and Servicing Medical Devices |
| US20080140106A1 (en) * | 2006-12-12 | 2008-06-12 | Kimberly-Clark Worldwide, Inc. | Enhanced cuff sealing for endotracheal tubes |
| US20080215136A1 (en) * | 2006-12-26 | 2008-09-04 | Gregorich Daniel J | Differential drug release from a medical device |
| US20080248079A1 (en) * | 2005-07-05 | 2008-10-09 | Ryan Dempsey | Tissue augmentation devices and methods |
| US20080287986A1 (en) * | 2007-05-18 | 2008-11-20 | Possis Medical, Inc. | Closure device |
| US20080312740A1 (en) * | 2003-02-10 | 2008-12-18 | Jurgen Wachter | Metal alloy for medical devices and implants |
| WO2008157285A1 (en) | 2007-06-13 | 2008-12-24 | Fmc Corporation | Biopolymer based implantable degradable devices |
| US20080318022A1 (en) * | 2001-10-30 | 2008-12-25 | Colorado State University Research Foundation (Csurf), Colorado Non-Profit | Outer layer material having entanglement of hydrophobic polymer host blended with a maleated hydrophobic polymer co-host, and hydrophilic polymer guest |
| US20090142385A1 (en) * | 2007-12-04 | 2009-06-04 | Warsaw Orthopedic, Inc. | Compositions for treating bone defects |
| US20090248141A1 (en) * | 2006-03-30 | 2009-10-01 | The Regents Of The University Of Colorado | Shape Memory Polymer Medical Devices |
| US20090246244A1 (en) * | 2008-03-27 | 2009-10-01 | Warsaw Orthopedic, Inc. | Malleable multi-component implants and materials therefor |
| WO2009131434A1 (en) * | 2008-04-24 | 2009-10-29 | Universiti Malaya | Extracellular scaffolds comprising blends of polyvinyl alcohol and carboxymethyl chitosan |
| WO2009137715A2 (en) * | 2008-05-07 | 2009-11-12 | Board Of Regents, The University Of Texas System | Versatile biodegradable elastic polymers featured with dual crosslinking mechanism for biomedical applications |
| US20090304644A1 (en) * | 2006-05-30 | 2009-12-10 | Cytori Therapeutics, Inc. | Systems and methods for manipulation of regenerative cells separated and concentrated from adipose tissue |
| US20100015104A1 (en) * | 2006-07-26 | 2010-01-21 | Cytori Therapeutics, Inc | Generation of adipose tissue and adipocytes |
| US7651684B2 (en) | 2001-12-07 | 2010-01-26 | Cytori Therapeutics, Inc. | Methods of using adipose tissue-derived cells in augmenting autologous fat transfer |
| US20100174367A1 (en) * | 2009-01-08 | 2010-07-08 | Bio Dg, Inc | Implantable medical devices comprising bio-degradable alloys |
| US20100209474A1 (en) * | 2006-05-01 | 2010-08-19 | Warsaw Orthopedic, Inc. | Malleable implants containing demineralized bone matrix |
| US20100209470A1 (en) * | 2006-05-01 | 2010-08-19 | Warsaw Orthopedic, Inc. An Indiana Corporation | Demineralized bone matrix devices |
| US20100255115A1 (en) * | 2006-05-01 | 2010-10-07 | Warsaw Orthopedic, Inc. | Bone filler material |
| US20100279405A1 (en) * | 2009-05-01 | 2010-11-04 | Alvin Peterson | Systems, methods and compositions for optimizing tissue and cell enriched grafts |
| WO2010138212A2 (en) * | 2009-05-29 | 2010-12-02 | Incube Labs, Llc | Biodegradable medical implants, polymer compositions and methods of use |
| US20110008442A1 (en) * | 2008-02-26 | 2011-01-13 | Board Of Regents, The University Of Texas System | Dendritic Macroporous Hydrogels Prepared by Crystal Templating |
| US20110052503A1 (en) * | 2007-12-21 | 2011-03-03 | Iopharma Technologies Ab | Biodegradable contrast agents |
| US7906132B2 (en) | 2002-09-17 | 2011-03-15 | Biocer-Entwickslung GmbH | Anti-infectious, biocompatible titanium coating for implants, and method for the production thereof |
| US20110125287A1 (en) * | 2009-11-24 | 2011-05-26 | Tyco Healthcare Group LP, New Haven CT and Confluent Surgical, Inc. | Reinforced Tissue Patch |
| US20110130774A1 (en) * | 2009-11-30 | 2011-06-02 | Tyco Healthcare Group Lp | Ventral Hernia Repair With Barbed Suture |
| EP2336318A1 (en) | 2002-11-13 | 2011-06-22 | Genzyme Corporation | Antisense modulation of apolipoprotein B expression |
| EP2336319A1 (en) | 2002-11-13 | 2011-06-22 | Genzyme Corporation | Antisense modulation of apolipoprotein B expression |
| US20110206646A1 (en) * | 2008-08-19 | 2011-08-25 | Zeni Alfonso | Methods of using adipose tissue-derived cells in the treatment of the lymphatic system and malignant disease |
| US8083726B1 (en) | 2005-09-30 | 2011-12-27 | Advanced Cardiovascular Systems, Inc. | Encapsulating cells and lumen |
| US8123739B2 (en) | 2008-08-19 | 2012-02-28 | Cook Medical Technologies Llc | Drainage catheter and method for catheterizing a patient |
| US20120178874A1 (en) * | 2005-10-31 | 2012-07-12 | Epstein Scott M | Structural hydrogel polymer device |
| US20120276221A1 (en) * | 2007-12-04 | 2012-11-01 | Boston Scientific Scimed, Inc. | Biodisintegrable medical devices |
| EP2559408A2 (en) | 2006-10-25 | 2013-02-20 | Biosensors International Group, Ltd. | Temporary intraluminal stent and methods of manufacture and use. |
| EP2600800A1 (en) * | 2010-08-05 | 2013-06-12 | Taris Biomedical, Inc. | Ureteral stent drug delivery device, kit, and method |
| US20140005606A1 (en) * | 2012-06-29 | 2014-01-02 | Mei-Chin Chen | Embeddable micro-needle patch for transdermal drug delivery and method of manufacturing the same |
| WO2014120587A1 (en) * | 2013-01-30 | 2014-08-07 | Boston Scientific Scimed, Inc. | Ureteral stent with drug-releasing structure |
| WO2014153144A1 (en) | 2013-03-14 | 2014-09-25 | Radisch Herbert R | Implantable medical devices comprising bio-degradable alloys with enhanced degradation rates |
| US8946194B2 (en) | 2010-10-08 | 2015-02-03 | Board Of Regents, University Of Texas System | One-step processing of hydrogels for mechanically robust and chemically desired features |
| US8968927B2 (en) | 2011-07-15 | 2015-03-03 | Covidien Lp | Degradable implantable battery |
| US8968926B2 (en) | 2011-07-15 | 2015-03-03 | Covidien Lp | Degradable implantable galvanic power source |
| EP2870981A1 (en) | 2013-11-12 | 2015-05-13 | Covidien LP | Degradable implantable battery |
| US9095558B2 (en) | 2010-10-08 | 2015-08-04 | Board Of Regents, The University Of Texas System | Anti-adhesive barrier membrane using alginate and hyaluronic acid for biomedical applications |
| WO2015191853A1 (en) * | 2014-06-13 | 2015-12-17 | Siemens Healthcare Diagnostics Inc. | Sample delivery system |
| WO2016033424A1 (en) | 2014-08-29 | 2016-03-03 | Genzyme Corporation | Methods for the prevention and treatment of major adverse cardiovascular events using compounds that modulate apolipoprotein b |
| US9427493B2 (en) | 2011-03-07 | 2016-08-30 | The Regents Of The University Of Colorado | Shape memory polymer intraocular lenses |
| WO2016181371A1 (en) * | 2015-05-14 | 2016-11-17 | Association For The Advancement Of Tissue Engineering And Cell Based Technologies And Therapies - A4Tec | An ureteral stent, methods and uses thereof |
| US9597395B2 (en) | 2001-12-07 | 2017-03-21 | Cytori Therapeutics, Inc. | Methods of using adipose tissue-derived cells in the treatment of cardiovascular conditions |
| US9662424B2 (en) | 2012-12-11 | 2017-05-30 | Board Of Regents, The University Of Texas System | Hydrogel membrane for adhesion prevention |
| US9731045B2 (en) | 2005-04-01 | 2017-08-15 | The Regents Of The University Of Colorado | Shape memory polymer |
| US9808252B2 (en) | 2009-04-02 | 2017-11-07 | Endoshape, Inc. | Vascular occlusion devices |
| US10010400B2 (en) | 2015-03-30 | 2018-07-03 | Taris Biomedical Llc | Devices and methods for local delivery of drug to upper urinary tract |
| CN109195642A (en) * | 2016-06-07 | 2019-01-11 | 医药研究产品有限公司 | Tissue enhancing filled compositions comprising nucleic acid, chitosan and hyaluronic acid and preparation method thereof |
| US10201351B2 (en) | 2011-09-30 | 2019-02-12 | Endoshape, Inc. | Continuous embolic coil and methods and devices for delivery of the same |
| US10286199B2 (en) | 2013-03-15 | 2019-05-14 | Taris Biomedical Llc | Drug delivery devices with drug-permeable component and methods |
| CN110721391A (en) * | 2019-09-30 | 2020-01-24 | 深圳泰睿仕医疗科技有限公司 | Disposable ureter catheterization support tube |
| US10603043B2 (en) | 2012-01-17 | 2020-03-31 | Endoshape, Inc. | Occlusion device for a vascular or biological lumen |
| CN111511310A (en) * | 2017-12-22 | 2020-08-07 | 聚合-医药有限公司 | Tubular implant with controlled biodegradation |
| US10894150B2 (en) | 2015-04-23 | 2021-01-19 | Taris Biomedical Llc | Drug delivery devices with drug-permeable component and methods |
| WO2021148564A1 (en) * | 2020-01-22 | 2021-07-29 | Dsm Ip Assets B.V. | Method of cross-linking biomaterial with a polyfunctional aziridine compound and products obtained therewith |
| CN115141447A (en) * | 2021-03-30 | 2022-10-04 | 合肥杰事杰新材料股份有限公司 | Antibacterial poly (hydroxyethyl methacrylate) material and preparation method and application thereof |
| US11565027B2 (en) | 2012-12-11 | 2023-01-31 | Board Of Regents, The University Of Texas System | Hydrogel membrane for adhesion prevention |
| US11896505B2 (en) | 2005-10-31 | 2024-02-13 | Scott M. Epstein | Methods for making and using a structural hydrogel polymer device |
Citations (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2485512A (en) | 1941-10-21 | 1949-10-18 | Alginate Ind Ltd | Manufacture of transparent alginic films |
| US2541804A (en) | 1949-08-04 | 1951-02-13 | Courtaulds Ltd | Production of artificial protein fibers |
| US2712672A (en) | 1952-01-28 | 1955-07-12 | Calcagno Luigi | Process for preparing proteic sponges |
| US2847713A (en) | 1955-05-13 | 1958-08-19 | Weingand Richard | Process for producing synthetic sausage skins and other laminar structures from alginates |
| US2897547A (en) | 1955-05-27 | 1959-08-04 | Weingand Richard | Process for producing synthetic sausage casing from alginates or alginic acid |
| US3271496A (en) | 1964-01-27 | 1966-09-06 | Amicon Corp | Method of shaping polyelectrolyte polymer |
| DE2827289A1 (en) | 1977-06-24 | 1979-01-11 | Ethicon Inc | MIXED POLYMERIZES OF LACTIDES AND GLYCOLIDES AND PROCESS FOR THEIR PRODUCTION |
| US4286341A (en) | 1979-04-16 | 1981-09-01 | Iowa State University Research Foundation, Inc. | Vascular prosthesis and method of making the same |
| US4339295A (en) | 1978-12-20 | 1982-07-13 | The United States Of America As Represented By The Secretary Of The Department Of Health & Human Services | Hydrogel adhesives and sandwiches or laminates using microwave energy |
| EP0065884A1 (en) | 1981-05-27 | 1982-12-01 | Unitika Ltd. | Urethral catheter capable of preventing urinary tract infection and process for producing the same |
| EP0080254A2 (en) | 1981-08-18 | 1983-06-01 | Fujitsu Limited | Transistor-transistor logic circuit |
| US4439322A (en) | 1980-07-02 | 1984-03-27 | Toray Industries, Inc. | Polymethyl methacrylate membrane |
| GB2151244A (en) | 1983-12-15 | 1985-07-17 | Biomatrix Inc | Insolubilized biocompatible hyaluronic acid preparations |
| US4582865A (en) | 1984-12-06 | 1986-04-15 | Biomatrix, Inc. | Cross-linked gels of hyaluronic acid and products containing such gels |
| US4605691A (en) | 1984-12-06 | 1986-08-12 | Biomatrix, Inc. | Cross-linked gels of hyaluronic acid and products containing such gels |
| US4613517A (en) | 1983-04-27 | 1986-09-23 | Becton, Dickinson And Company | Heparinization of plasma treated surfaces |
| US4636524A (en) | 1984-12-06 | 1987-01-13 | Biomatrix, Inc. | Cross-linked gels of hyaluronic acid and products containing such gels |
| EP0213908A2 (en) | 1985-08-26 | 1987-03-11 | Hana Biologics, Inc. | Transplantable artificial tissue and process |
| US4650488A (en) | 1984-05-16 | 1987-03-17 | Richards Medical Company | Biodegradable prosthetic device |
| US4716224A (en) | 1984-05-04 | 1987-12-29 | Seikagaku Kogyo Co. Ltd. | Crosslinked hyaluronic acid and its use |
| US4716154A (en) | 1984-06-08 | 1987-12-29 | Pharmacia Ab | Gel of crosslinked hyaluronic acid for use as a vitreous humor substitute |
| EP0271216A2 (en) | 1986-11-10 | 1988-06-15 | Ube-Nitto Kasei Co. Ltd. | Synthetic vascular prosthesis and method for manufacturing same |
| US4801475A (en) | 1984-08-23 | 1989-01-31 | Gregory Halpern | Method of hydrophilic coating of plastics |
| US4814120A (en) | 1984-02-21 | 1989-03-21 | Bioetica S.A. | Process for the preparation of collagen tubes |
| US4838876A (en) | 1986-04-29 | 1989-06-13 | The Kendall Company | Silicone rubber catheter having improved surface morphology |
| WO1989005671A1 (en) | 1987-12-23 | 1989-06-29 | Bard Limited | Catheter |
| US4851521A (en) | 1985-07-08 | 1989-07-25 | Fidia, S.P.A. | Esters of hyaluronic acid |
| US4863907A (en) | 1984-06-29 | 1989-09-05 | Seikagaku Kogyo Co., Ltd. | Crosslinked glycosaminoglycans and their use |
| EP0341745A1 (en) | 1988-05-13 | 1989-11-15 | FIDIA S.p.A. | Crosslinked carboxy polysaccharides |
| US4888016A (en) | 1988-02-10 | 1989-12-19 | Langerman David W | "Spare parts" for use in ophthalmic surgical procedures |
| US4902295A (en) | 1985-08-26 | 1990-02-20 | Hana Biologics, Inc. | Transplantable artificial tissue |
| US4906237A (en) | 1985-09-13 | 1990-03-06 | Astra Meditec Ab | Method of forming an improved hydrophilic coating on a polymer surface |
| US4923645A (en) | 1987-11-16 | 1990-05-08 | Damon Biotech, Inc. | Sustained release of encapsulated molecules |
| WO1990010020A1 (en) | 1989-02-24 | 1990-09-07 | Chemical Works Of Gedeon Richter Ltd. | Novel compositions containing hyaluronic acid associates and a process for preparing same |
| US4957744A (en) | 1986-10-13 | 1990-09-18 | Fidia, S.P.A. | Cross-linked esters of hyaluronic acid |
| US4997443A (en) | 1985-08-26 | 1991-03-05 | Hana Biologics, Inc. | Transplantable artificial tissue and process |
| WO1991007200A1 (en) | 1989-11-13 | 1991-05-30 | Boston Scientific Corporation | Catheter with dissolvable tip |
| US5057606A (en) | 1989-01-24 | 1991-10-15 | Minnesota Mining And Manufacturing Company | Form-in-place polysaccharide gels |
| US5061738A (en) | 1988-04-18 | 1991-10-29 | Becton, Dickinson And Company | Blood compatible, lubricious article and composition and method therefor |
| EP0454373A2 (en) | 1990-04-23 | 1991-10-30 | Monsanto Company | Gellan gum fibers |
| US5077352A (en) | 1990-04-23 | 1991-12-31 | C. R. Bard, Inc. | Flexible lubricious organic coatings |
| US5085629A (en) | 1988-10-06 | 1992-02-04 | Medical Engineering Corporation | Biodegradable stent |
| US5089606A (en) | 1989-01-24 | 1992-02-18 | Minnesota Mining And Manufacturing Company | Water-insoluble polysaccharide hydrogel foam for medical applications |
| JPH04145218A (en) | 1990-10-08 | 1992-05-19 | Canon Inc | Static-pressure bearing |
| US5128326A (en) | 1984-12-06 | 1992-07-07 | Biomatrix, Inc. | Drug delivery systems based on hyaluronans derivatives thereof and their salts and methods of producing same |
| WO1992013579A1 (en) | 1991-02-11 | 1992-08-20 | Fidia S.P.A. | Biodegradable and bioabsorbable guide channels for use in nerve treatment and regeneration |
| US5149543A (en) | 1990-10-05 | 1992-09-22 | Massachusetts Institute Of Technology | Ionically cross-linked polymeric microcapsules |
| EP0507604A2 (en) | 1991-04-05 | 1992-10-07 | Lifecore Biomedical, Inc. | Ionically crosslinked carboxyl-containing polysaccharides for adhesion prevention |
| WO1992018098A1 (en) | 1991-04-10 | 1992-10-29 | Capelli Christopher C | Antimicrobial compositions useful for medical applications |
| US5202431A (en) | 1985-07-08 | 1993-04-13 | Fidia, S.P.A. | Partial esters of hyaluronic acid |
| WO1993009176A2 (en) | 1991-10-29 | 1993-05-13 | Clover Consolidated, Limited | Crosslinkable polysaccharides, polycations and lipids useful for encapsulation and drug release |
| US5278201A (en) | 1988-10-03 | 1994-01-11 | Atrix Laboratories, Inc. | Biodegradable in-situ forming implants and methods of producing the same |
| US5298569A (en) | 1985-10-30 | 1994-03-29 | Nippon Paint Co. | Metallic ester acrylic compositions capable of releasing bioactive substance at a controlled rate |
| US5302393A (en) | 1991-07-11 | 1994-04-12 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Method for inhibiting biological degradation of implantation polymeric material, inhibitor thereof and implantation polymeric material containing the inhibitor |
| US5306764A (en) | 1992-09-03 | 1994-04-26 | China Technical Consultants Inc. | Water dispersible polyurethane and process for preparation thereof |
| US5322935A (en) | 1993-04-27 | 1994-06-21 | Alliedsignal Inc. | Rigid materials having high surface area and low density |
| US5334640A (en) | 1992-04-08 | 1994-08-02 | Clover Consolidated, Ltd. | Ionically covalently crosslinked and crosslinkable biocompatible encapsulation compositions and methods |
| EP0645150A1 (en) | 1993-09-29 | 1995-03-29 | Hercules Incorporated | Medical devices subject to triggered disintegration |
| US5413782A (en) | 1990-12-19 | 1995-05-09 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Binding pharmaceuticals to ion exchange resins |
| US5514377A (en) | 1994-03-08 | 1996-05-07 | The Regents Of The University Of California | In situ dissolution of alginate coatings of biological tissue transplants |
| US5532305A (en) | 1993-07-26 | 1996-07-02 | Shiseido Company Ltd. | Controlled release preparation for bioactive substances |
| US5554388A (en) | 1989-02-25 | 1996-09-10 | Danbiosyst Uk Limited | Systemic drug delivery compositions comprising a polycationi substance |
| US5736595A (en) * | 1993-05-03 | 1998-04-07 | Chemische Fabrik Stockhausen Gmbh | Polymer composition, absorbent material composition, their production and their use |
-
2000
- 2000-02-25 US US09/512,698 patent/US6368356B1/en not_active Expired - Lifetime
Patent Citations (73)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2485512A (en) | 1941-10-21 | 1949-10-18 | Alginate Ind Ltd | Manufacture of transparent alginic films |
| US2541804A (en) | 1949-08-04 | 1951-02-13 | Courtaulds Ltd | Production of artificial protein fibers |
| US2712672A (en) | 1952-01-28 | 1955-07-12 | Calcagno Luigi | Process for preparing proteic sponges |
| US2847713A (en) | 1955-05-13 | 1958-08-19 | Weingand Richard | Process for producing synthetic sausage skins and other laminar structures from alginates |
| US2897547A (en) | 1955-05-27 | 1959-08-04 | Weingand Richard | Process for producing synthetic sausage casing from alginates or alginic acid |
| US3271496A (en) | 1964-01-27 | 1966-09-06 | Amicon Corp | Method of shaping polyelectrolyte polymer |
| DE2827289A1 (en) | 1977-06-24 | 1979-01-11 | Ethicon Inc | MIXED POLYMERIZES OF LACTIDES AND GLYCOLIDES AND PROCESS FOR THEIR PRODUCTION |
| US4137921A (en) | 1977-06-24 | 1979-02-06 | Ethicon, Inc. | Addition copolymers of lactide and glycolide and method of preparation |
| US4339295A (en) | 1978-12-20 | 1982-07-13 | The United States Of America As Represented By The Secretary Of The Department Of Health & Human Services | Hydrogel adhesives and sandwiches or laminates using microwave energy |
| US4286341A (en) | 1979-04-16 | 1981-09-01 | Iowa State University Research Foundation, Inc. | Vascular prosthesis and method of making the same |
| US4439322A (en) | 1980-07-02 | 1984-03-27 | Toray Industries, Inc. | Polymethyl methacrylate membrane |
| EP0065884A1 (en) | 1981-05-27 | 1982-12-01 | Unitika Ltd. | Urethral catheter capable of preventing urinary tract infection and process for producing the same |
| EP0080254A2 (en) | 1981-08-18 | 1983-06-01 | Fujitsu Limited | Transistor-transistor logic circuit |
| US4613517A (en) | 1983-04-27 | 1986-09-23 | Becton, Dickinson And Company | Heparinization of plasma treated surfaces |
| GB2151244A (en) | 1983-12-15 | 1985-07-17 | Biomatrix Inc | Insolubilized biocompatible hyaluronic acid preparations |
| US4814120A (en) | 1984-02-21 | 1989-03-21 | Bioetica S.A. | Process for the preparation of collagen tubes |
| US4716224A (en) | 1984-05-04 | 1987-12-29 | Seikagaku Kogyo Co. Ltd. | Crosslinked hyaluronic acid and its use |
| US4650488A (en) | 1984-05-16 | 1987-03-17 | Richards Medical Company | Biodegradable prosthetic device |
| US4716154A (en) | 1984-06-08 | 1987-12-29 | Pharmacia Ab | Gel of crosslinked hyaluronic acid for use as a vitreous humor substitute |
| US4863907A (en) | 1984-06-29 | 1989-09-05 | Seikagaku Kogyo Co., Ltd. | Crosslinked glycosaminoglycans and their use |
| US4801475A (en) | 1984-08-23 | 1989-01-31 | Gregory Halpern | Method of hydrophilic coating of plastics |
| US4582865A (en) | 1984-12-06 | 1986-04-15 | Biomatrix, Inc. | Cross-linked gels of hyaluronic acid and products containing such gels |
| US4636524A (en) | 1984-12-06 | 1987-01-13 | Biomatrix, Inc. | Cross-linked gels of hyaluronic acid and products containing such gels |
| US4605691A (en) | 1984-12-06 | 1986-08-12 | Biomatrix, Inc. | Cross-linked gels of hyaluronic acid and products containing such gels |
| US5128326A (en) | 1984-12-06 | 1992-07-07 | Biomatrix, Inc. | Drug delivery systems based on hyaluronans derivatives thereof and their salts and methods of producing same |
| US4965353A (en) | 1985-07-08 | 1990-10-23 | Fidia S.P.A. | Polysaccharide esters and their salts |
| US5202431A (en) | 1985-07-08 | 1993-04-13 | Fidia, S.P.A. | Partial esters of hyaluronic acid |
| US4851521A (en) | 1985-07-08 | 1989-07-25 | Fidia, S.P.A. | Esters of hyaluronic acid |
| US4997443A (en) | 1985-08-26 | 1991-03-05 | Hana Biologics, Inc. | Transplantable artificial tissue and process |
| EP0213908A2 (en) | 1985-08-26 | 1987-03-11 | Hana Biologics, Inc. | Transplantable artificial tissue and process |
| US4902295A (en) | 1985-08-26 | 1990-02-20 | Hana Biologics, Inc. | Transplantable artificial tissue |
| US4906237A (en) | 1985-09-13 | 1990-03-06 | Astra Meditec Ab | Method of forming an improved hydrophilic coating on a polymer surface |
| US5298569A (en) | 1985-10-30 | 1994-03-29 | Nippon Paint Co. | Metallic ester acrylic compositions capable of releasing bioactive substance at a controlled rate |
| US4838876A (en) | 1986-04-29 | 1989-06-13 | The Kendall Company | Silicone rubber catheter having improved surface morphology |
| US4957744A (en) | 1986-10-13 | 1990-09-18 | Fidia, S.P.A. | Cross-linked esters of hyaluronic acid |
| US4941870A (en) | 1986-11-10 | 1990-07-17 | Ube-Nitto Kasei Co., Ltd. | Method for manufacturing a synthetic vascular prosthesis |
| US4878907A (en) | 1986-11-10 | 1989-11-07 | Ube-Nitto Kasei Co., Ltd. | Synthetic vascular prosthesis |
| EP0271216A2 (en) | 1986-11-10 | 1988-06-15 | Ube-Nitto Kasei Co. Ltd. | Synthetic vascular prosthesis and method for manufacturing same |
| US4923645A (en) | 1987-11-16 | 1990-05-08 | Damon Biotech, Inc. | Sustained release of encapsulated molecules |
| WO1989005671A1 (en) | 1987-12-23 | 1989-06-29 | Bard Limited | Catheter |
| US4888016A (en) | 1988-02-10 | 1989-12-19 | Langerman David W | "Spare parts" for use in ophthalmic surgical procedures |
| US5061738A (en) | 1988-04-18 | 1991-10-29 | Becton, Dickinson And Company | Blood compatible, lubricious article and composition and method therefor |
| EP0341745A1 (en) | 1988-05-13 | 1989-11-15 | FIDIA S.p.A. | Crosslinked carboxy polysaccharides |
| US5278201A (en) | 1988-10-03 | 1994-01-11 | Atrix Laboratories, Inc. | Biodegradable in-situ forming implants and methods of producing the same |
| US5085629A (en) | 1988-10-06 | 1992-02-04 | Medical Engineering Corporation | Biodegradable stent |
| US5057606A (en) | 1989-01-24 | 1991-10-15 | Minnesota Mining And Manufacturing Company | Form-in-place polysaccharide gels |
| US5089606A (en) | 1989-01-24 | 1992-02-18 | Minnesota Mining And Manufacturing Company | Water-insoluble polysaccharide hydrogel foam for medical applications |
| WO1990010020A1 (en) | 1989-02-24 | 1990-09-07 | Chemical Works Of Gedeon Richter Ltd. | Novel compositions containing hyaluronic acid associates and a process for preparing same |
| US5554388A (en) | 1989-02-25 | 1996-09-10 | Danbiosyst Uk Limited | Systemic drug delivery compositions comprising a polycationi substance |
| WO1991007200A1 (en) | 1989-11-13 | 1991-05-30 | Boston Scientific Corporation | Catheter with dissolvable tip |
| US5077352A (en) | 1990-04-23 | 1991-12-31 | C. R. Bard, Inc. | Flexible lubricious organic coatings |
| EP0454373A2 (en) | 1990-04-23 | 1991-10-30 | Monsanto Company | Gellan gum fibers |
| US5149543A (en) | 1990-10-05 | 1992-09-22 | Massachusetts Institute Of Technology | Ionically cross-linked polymeric microcapsules |
| US5308701A (en) | 1990-10-05 | 1994-05-03 | Smadar Cohen | Ionically cross-linked polymeric microcapsules |
| JPH04145218A (en) | 1990-10-08 | 1992-05-19 | Canon Inc | Static-pressure bearing |
| US5413782A (en) | 1990-12-19 | 1995-05-09 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Binding pharmaceuticals to ion exchange resins |
| WO1992013579A1 (en) | 1991-02-11 | 1992-08-20 | Fidia S.P.A. | Biodegradable and bioabsorbable guide channels for use in nerve treatment and regeneration |
| EP0507604A2 (en) | 1991-04-05 | 1992-10-07 | Lifecore Biomedical, Inc. | Ionically crosslinked carboxyl-containing polysaccharides for adhesion prevention |
| WO1992018098A1 (en) | 1991-04-10 | 1992-10-29 | Capelli Christopher C | Antimicrobial compositions useful for medical applications |
| US5302393A (en) | 1991-07-11 | 1994-04-12 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Method for inhibiting biological degradation of implantation polymeric material, inhibitor thereof and implantation polymeric material containing the inhibitor |
| WO1993009176A2 (en) | 1991-10-29 | 1993-05-13 | Clover Consolidated, Limited | Crosslinkable polysaccharides, polycations and lipids useful for encapsulation and drug release |
| US5334640A (en) | 1992-04-08 | 1994-08-02 | Clover Consolidated, Ltd. | Ionically covalently crosslinked and crosslinkable biocompatible encapsulation compositions and methods |
| US5306764A (en) | 1992-09-03 | 1994-04-26 | China Technical Consultants Inc. | Water dispersible polyurethane and process for preparation thereof |
| US5328939A (en) * | 1993-04-27 | 1994-07-12 | Alliedsignal Inc. | Rigid materials having high surface area and low density |
| US5322935A (en) | 1993-04-27 | 1994-06-21 | Alliedsignal Inc. | Rigid materials having high surface area and low density |
| US5736595A (en) * | 1993-05-03 | 1998-04-07 | Chemische Fabrik Stockhausen Gmbh | Polymer composition, absorbent material composition, their production and their use |
| US5532305A (en) | 1993-07-26 | 1996-07-02 | Shiseido Company Ltd. | Controlled release preparation for bioactive substances |
| EP0645150A1 (en) | 1993-09-29 | 1995-03-29 | Hercules Incorporated | Medical devices subject to triggered disintegration |
| US5531716A (en) | 1993-09-29 | 1996-07-02 | Hercules Incorporated | Medical devices subject to triggered disintegration |
| US5650116A (en) * | 1993-09-29 | 1997-07-22 | Hercules Incorporated | Method of making a medical device from ironically crosslinked polymer |
| US6096018A (en) * | 1993-09-29 | 2000-08-01 | Scimed Life Systems, Inc. | Medical treatment utilizing ionically crosslinked medical devices |
| US6126645A (en) * | 1993-09-29 | 2000-10-03 | Scimed Life Systems, Inc. | Medical devices subject to triggered disintegration |
| US5514377A (en) | 1994-03-08 | 1996-05-07 | The Regents Of The University Of California | In situ dissolution of alginate coatings of biological tissue transplants |
Non-Patent Citations (3)
| Title |
|---|
| Andrade, Joseph D., "Hydrogels for Medical and Related Applications", Aug. 27-28, 1975, pp. 1-36 (Editor, presented article at that time); AM. Chem. Soc. 1976. |
| Kocvara et al., "Gel-Fabric Prostheses of the Ureter", Journal of Biomedical Research, vol. 1, pp. 325-336 (1967). |
| Ross, "Living Cure" (Science and the Citizen), Scientific American, Jun. 1993, pp. 18-23. |
Cited By (254)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6468312B1 (en) * | 1998-07-26 | 2002-10-22 | Klaus Rennebeck | Ureapoietic organ replacement |
| US20040151752A1 (en) * | 1999-04-12 | 2004-08-05 | Chee-Youb Won | Hydrogel entrapping therapeutic agent and stent with coating comprising this |
| US7138132B2 (en) | 1999-04-12 | 2006-11-21 | Cornell Research Foundation, Inc. | Hydrogel entrapping therapeutic agent and stent with coating comprising this |
| US6905700B2 (en) * | 1999-04-12 | 2005-06-14 | Cornell Research Foundation, Inc. | Hydrogel entrapping therapeutic agent and stent with coating comprising this |
| US6921819B2 (en) * | 2000-07-19 | 2005-07-26 | Laboratoires D'esthetique Appliquee | Polysaccharide crosslinking, hydrogel preparation, resulting polysaccharide(s) and hydrogel(s), uses thereof |
| US20030148995A1 (en) * | 2000-07-19 | 2003-08-07 | Estelle Piron | Polysaccharide crosslinking, hydrogel preparation, resulting polysaccharide(s) and hydrogel(s), uses thereof |
| US20050278024A1 (en) * | 2001-02-28 | 2005-12-15 | Gregory Murphy | Kit and method for use during ventricular restoration |
| US20020133143A1 (en) * | 2001-02-28 | 2002-09-19 | Gregory Murphy | Ventricular restoration shaping apparatus and method of use |
| US20030181940A1 (en) * | 2001-02-28 | 2003-09-25 | Gregory Murphy | Ventricular restoration shaping apparatus and method of use |
| US6959711B2 (en) | 2001-02-28 | 2005-11-01 | Chase Medical, L.P. | Kit and method for use during ventricular restoration |
| US20020133054A1 (en) * | 2001-02-28 | 2002-09-19 | Gregory Murphy | Sizing apparatus and method for use during ventricular restoration |
| US6681773B2 (en) | 2001-02-28 | 2004-01-27 | Chase Medical, Inc. | Kit and method for use during ventricular restoration |
| US6702763B2 (en) | 2001-02-28 | 2004-03-09 | Chase Medical, L.P. | Sizing apparatus and method for use during ventricular restoration |
| US6994093B2 (en) | 2001-02-28 | 2006-02-07 | Chase Medical, L.P. | Ventricular restoration shaping apparatus and method of use |
| US20020133182A1 (en) * | 2001-02-28 | 2002-09-19 | Gregory Murphy | Kit and method for use during ventricular restoration |
| US20030068817A1 (en) * | 2001-04-30 | 2003-04-10 | Dan Gazit | Vascular tissue engineering |
| US8197553B2 (en) | 2001-04-30 | 2012-06-12 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Composite scaffolds and methods using same for generating complex tissue grafts |
| US20090035349A1 (en) * | 2001-04-30 | 2009-02-05 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Composite scaffolds and methods using same for generating complex tissue grafts |
| US20040203146A1 (en) * | 2001-04-30 | 2004-10-14 | Dan Gazit | Composite scaffolds and methods using same for generating complex tissue grafts |
| US6673453B2 (en) * | 2001-06-12 | 2004-01-06 | Biocoat Incorporated | Coatings appropriate for medical devices |
| US20110274821A1 (en) * | 2001-06-26 | 2011-11-10 | Biointeractions, Ltd. | Heparin coatings |
| US20030021762A1 (en) * | 2001-06-26 | 2003-01-30 | Luthra Ajay K. | Polysaccharide biomaterials and methods of use thereof |
| US9127091B2 (en) * | 2001-06-26 | 2015-09-08 | Biointeractions, Ltd. | Heparin coatings |
| US20040142016A1 (en) * | 2001-06-26 | 2004-07-22 | Biointeractions, Ltd., University Of Reading | Polysaccharide biomaterials and methods of use thereof |
| US8101196B2 (en) * | 2001-06-26 | 2012-01-24 | Biointeractions, Ltd. | Polysaccharide biomaterials and methods of use thereof |
| US20040249408A1 (en) * | 2001-09-05 | 2004-12-09 | Chase Medical, Lp | Method and device for endoscopic surgical ventricular repair |
| US7485088B2 (en) | 2001-09-05 | 2009-02-03 | Chase Medical L.P. | Method and device for percutaneous surgical ventricular repair |
| US8524886B2 (en) | 2001-10-30 | 2013-09-03 | Colorado State University Research Foundation | Outer layer having entanglement of hydrophobic polymer host and hydrophilic polymer guest |
| US9533077B2 (en) | 2001-10-30 | 2017-01-03 | Colorado State University Research Foundation | Outer layer having entanglement of hydrophobic polymer host blended with a maleated hydrophobic polymer co-host, and hydrophilic polymer guest |
| US9567447B2 (en) | 2001-10-30 | 2017-02-14 | Colorado State University Research Foundation | Outer layer having entanglement of hydrophobic polymer host and hydrophilic polymer guest |
| US10435523B2 (en) | 2001-10-30 | 2019-10-08 | Colorado State University Research Foundation | Material comprising outer layer having entanglement of hydrophobic polymer host blended with anhydride functionalized hydrophobic polymer co-host and hydrophilic guest |
| US8524884B2 (en) | 2001-10-30 | 2013-09-03 | Colorado State University Research Foundation | Outer layer material having entanglement of hydrophobic polymer hostblended with a maleated hydrophobic polymer co-host, and hydrophilic polymer guest |
| US20100150983A1 (en) * | 2001-10-30 | 2010-06-17 | Colorado State University Research Foundation | Outer layer having entanglement of hydrophobic polymer host and hydrophilic polymer guest |
| US20080318022A1 (en) * | 2001-10-30 | 2008-12-25 | Colorado State University Research Foundation (Csurf), Colorado Non-Profit | Outer layer material having entanglement of hydrophobic polymer host blended with a maleated hydrophobic polymer co-host, and hydrophilic polymer guest |
| US20030083433A1 (en) * | 2001-10-30 | 2003-05-01 | James Susan P. | Outer layer having entanglement of hydrophobic polymer host and hydrophilic polymer guest |
| US7662954B2 (en) | 2001-10-30 | 2010-02-16 | Colorado State University Research Foundation | Outer layer having entanglement of hydrophobic polymer host and hydrophilic polymer guest |
| US9597395B2 (en) | 2001-12-07 | 2017-03-21 | Cytori Therapeutics, Inc. | Methods of using adipose tissue-derived cells in the treatment of cardiovascular conditions |
| US9480718B2 (en) | 2001-12-07 | 2016-11-01 | Cytori Therapeutics, Inc. | Methods of using adipose-derived regenerative cells in the treatment of peripheral vascular disease and related disorders |
| US20100303774A1 (en) * | 2001-12-07 | 2010-12-02 | Cytori Therapeutics, Inc. | Methods of using regenerative cells in the treatment of musculoskeletal disorders |
| US8105580B2 (en) | 2001-12-07 | 2012-01-31 | Cytori Therapeutics, Inc. | Methods of using adipose derived stem cells to promote wound healing |
| US20050260174A1 (en) * | 2001-12-07 | 2005-11-24 | Fraser John K | Systems and methods for treating patients with processed lipoaspirate cells |
| US20050260175A1 (en) * | 2001-12-07 | 2005-11-24 | Hedrick Marc H | Systems and methods for isolating and using clinically safe adipose derived regenerative cells |
| US20100233139A1 (en) * | 2001-12-07 | 2010-09-16 | Hedrick Marc H | Methods of using adipose tissue-derived cells in augmenting autologous fat transfer |
| US8119121B2 (en) | 2001-12-07 | 2012-02-21 | Cytori Therapeutics, Inc. | Autologous adipose tissue implant with concentrated stem cells |
| US7771716B2 (en) | 2001-12-07 | 2010-08-10 | Cytori Therapeutics, Inc. | Methods of using regenerative cells in the treatment of musculoskeletal disorders |
| US8246947B2 (en) | 2001-12-07 | 2012-08-21 | Cytori Therapeutics, Inc. | Methods of using adipose tissue-derived cells in augmenting autologous fat transfer |
| US8337834B2 (en) | 2001-12-07 | 2012-12-25 | Cytori Therapeutics, Inc. | Methods of making enhanced, autologous fat grafts |
| US20060204556A1 (en) * | 2001-12-07 | 2006-09-14 | Cytori Therapeutics, Inc. | Cell-loaded prostheses for regenerative intraluminal applications |
| US20050095228A1 (en) * | 2001-12-07 | 2005-05-05 | Fraser John K. | Methods of using regenerative cells in the treatment of peripheral vascular disease and related disorders |
| US20050084961A1 (en) * | 2001-12-07 | 2005-04-21 | Hedrick Marc H. | Systems and methods for separating and concentrating regenerative cells from tissue |
| US8404229B2 (en) | 2001-12-07 | 2013-03-26 | Cytori Therapeutics, Inc. | Methods of using adipose derived stem cells to treat acute tubular necrosis |
| US9872877B2 (en) | 2001-12-07 | 2018-01-23 | Cytori Therapeutics, Inc. | Methods of using regenerative cells to promote epithelialization or neodermis formation |
| US9849149B2 (en) | 2001-12-07 | 2017-12-26 | Cytori Therapeutics, Inc. | Methods of using regenerative cells in the treatment of erectile dysfunction |
| US8691216B2 (en) | 2001-12-07 | 2014-04-08 | Cytori Therapeutics, Inc. | Methods of using regenerative cells to promote wound healing |
| US20050008626A1 (en) * | 2001-12-07 | 2005-01-13 | Fraser John K. | Methods of using adipose tissue-derived cells in the treatment of cardiovascular conditions |
| US20050058632A1 (en) * | 2001-12-07 | 2005-03-17 | Hedrick Marc H. | Cell carrier and cell carrier containment devices containing regenerative cells |
| US20050048035A1 (en) * | 2001-12-07 | 2005-03-03 | Fraser John K. | Methods of using regenerative cells in the treatment of stroke and related diseases and disorders |
| US7651684B2 (en) | 2001-12-07 | 2010-01-26 | Cytori Therapeutics, Inc. | Methods of using adipose tissue-derived cells in augmenting autologous fat transfer |
| US9511094B2 (en) | 2001-12-07 | 2016-12-06 | Cytori Therapeutics, Inc. | Methods of using regenerative cells in the treatment of stroke and related diseases and disorders |
| US8771678B2 (en) | 2001-12-07 | 2014-07-08 | Cytori Therapeutics, Inc. | Methods of using adipose tissue-derived cells in augmenting autologous fat transfer |
| US9511096B2 (en) | 2001-12-07 | 2016-12-06 | Cytori Therapeutics, Inc. | Methods of using regenerative cells to treat an ischemic wound |
| US20100015204A1 (en) * | 2001-12-07 | 2010-01-21 | Hedrick Marc H | Cell carrier and cell carrier containment devices containing regenerative cells |
| US20050048644A1 (en) * | 2001-12-07 | 2005-03-03 | Hedrick Marc H. | Methods of using regenerative cells in the treatment of musculoskeletal disorders |
| US9504716B2 (en) | 2001-12-07 | 2016-11-29 | Cytori Therapeutics, Inc. | Methods of using adipose derived regenerative cells to promote restoration of intevertebral disc |
| US8883499B2 (en) | 2001-12-07 | 2014-11-11 | Cytori Therapeutics, Inc. | Systems and methods for isolating and using clinically safe adipose derived regenerative cells |
| US9492483B2 (en) | 2001-12-07 | 2016-11-15 | Cytori Therapeutics, Inc. | Methods of using regenerative cells to treat a burn |
| US20050048033A1 (en) * | 2001-12-07 | 2005-03-03 | Fraser John K. | Methods of using regenerative cells in the treatment of renal diseases and disorders |
| US20090297488A1 (en) * | 2001-12-07 | 2009-12-03 | John K Fraser | Methods of using regenerative cells in the treatment of peripheral vascular disease and related disorders |
| US9198937B2 (en) | 2001-12-07 | 2015-12-01 | Cytori Therapeutics, Inc. | Adipose-derived regenerative cells for treating liver injury |
| US20050048036A1 (en) * | 2001-12-07 | 2005-03-03 | Hedrick Marc H. | Methods of using regenerative cells in the treatment of inherited and acquired disorders of the bone, bone marrow, liver, and other tissues |
| US20050048034A1 (en) * | 2001-12-07 | 2005-03-03 | Fraser John K. | Methods of using regenerative cells to promote wound healing |
| US9463203B2 (en) | 2001-12-07 | 2016-10-11 | Cytori Therapeutics, Inc. | Methods of using regenerative cells in the treatment of cartilage defects |
| US7514075B2 (en) | 2001-12-07 | 2009-04-07 | Cytori Therapeutics, Inc. | Systems and methods for separating and concentrating adipose derived stem cells from tissue |
| US7595043B2 (en) | 2001-12-07 | 2009-09-29 | Cytori Therapeutics, Inc. | Method for processing and using adipose-derived stem cells |
| US7585670B2 (en) | 2001-12-07 | 2009-09-08 | Cytori Therapeutics, Inc. | Automated methods for isolating and using clinically safe adipose derived regenerative cells |
| US20030204238A1 (en) * | 2002-04-26 | 2003-10-30 | Eugene Tedeschi | Coated stent with crimpable coating |
| US7470281B2 (en) * | 2002-04-26 | 2008-12-30 | Medtronic Vascular, Inc. | Coated stent with crimpable coating |
| US7906132B2 (en) | 2002-09-17 | 2011-03-15 | Biocer-Entwickslung GmbH | Anti-infectious, biocompatible titanium coating for implants, and method for the production thereof |
| WO2004026360A3 (en) * | 2002-09-19 | 2004-07-22 | Medtronic Inc | A medical assembly suitable for long-term implantation and method for fabricating the same |
| US7025982B2 (en) | 2002-09-19 | 2006-04-11 | Medtronic, Inc. | Medical assembly suitable for long-term implantation and method for fabricating the same |
| US7919600B2 (en) | 2002-09-23 | 2011-04-05 | Alcatel-Lucent Usa Inc. | Compositions that reversibly gel and de-gel |
| US20040059102A1 (en) * | 2002-09-23 | 2004-03-25 | Lucent Technologies Inc. | Compositions that reversibly gel and de-gel |
| US20040062808A1 (en) * | 2002-09-23 | 2004-04-01 | Lucent Technologies Inc. | Agent delivery system capable of selectively releasing an agent |
| US8389700B2 (en) | 2002-09-23 | 2013-03-05 | Alcatel Lucent | Agent delivery system capable of selectively releasing an agent |
| EP2336319A1 (en) | 2002-11-13 | 2011-06-22 | Genzyme Corporation | Antisense modulation of apolipoprotein B expression |
| EP2336318A1 (en) | 2002-11-13 | 2011-06-22 | Genzyme Corporation | Antisense modulation of apolipoprotein B expression |
| US6992127B2 (en) * | 2002-11-25 | 2006-01-31 | Ast Products, Inc. | Polymeric coatings containing a pH buffer agent |
| WO2004048455A1 (en) * | 2002-11-25 | 2004-06-10 | Ast Products, Inc. | POLYMERIC COATINGS CONTAINING A pH BUFFER AGENT |
| US20040102558A1 (en) * | 2002-11-25 | 2004-05-27 | Tung-Liang Lin | Polymeric coatings containing a pH buffer agent |
| US20080312740A1 (en) * | 2003-02-10 | 2008-12-18 | Jurgen Wachter | Metal alloy for medical devices and implants |
| US20100222866A1 (en) * | 2003-02-10 | 2010-09-02 | Jurgen Wachter | Metal alloy for medical devices and implants |
| US8349249B2 (en) | 2003-02-10 | 2013-01-08 | Heraeus Precious Metals Gmbh & Co. Kg | Metal alloy for medical devices and implants |
| US8403980B2 (en) * | 2003-02-10 | 2013-03-26 | Heraeus Materials Technology Gmbh & Co. Kg | Metal alloy for medical devices and implants |
| US7390525B2 (en) | 2003-05-06 | 2008-06-24 | Boston Scientific Scimed, Inc. | Processes for producing polymer coatings for release of therapeutic agent |
| US20050158359A1 (en) * | 2003-05-06 | 2005-07-21 | Epstein Samuel J. | Processes for producing polymer coatings for release of therapeutic agent |
| US6923996B2 (en) | 2003-05-06 | 2005-08-02 | Scimed Life Systems, Inc. | Processes for producing polymer coatings for release of therapeutic agent |
| US20050020506A1 (en) * | 2003-07-25 | 2005-01-27 | Drapeau Susan J. | Crosslinked compositions comprising collagen and demineralized bone matrix, methods of making and methods of use |
| US20050240277A1 (en) * | 2004-04-26 | 2005-10-27 | Peter Aliski | Stent with flexible elements |
| US20050240280A1 (en) * | 2004-04-26 | 2005-10-27 | Peter Aliski | Ureteral stent |
| US20050240278A1 (en) * | 2004-04-26 | 2005-10-27 | Peter Aliski | Stent improvements |
| US7507218B2 (en) | 2004-04-26 | 2009-03-24 | Gyrus Acmi, Inc. | Stent with flexible elements |
| US7470247B2 (en) | 2004-04-26 | 2008-12-30 | Gyrus Acmi, Inc. | Ureteral stent |
| WO2006069349A3 (en) * | 2004-12-22 | 2007-08-09 | Cytori Therapeutics Inc | Cell-loaded prostheses for regenerative intraluminal applications |
| WO2006069349A2 (en) * | 2004-12-22 | 2006-06-29 | Cytori Therapeutics, Inc. | Cell-loaded prostheses for regenerative intraluminal applications |
| US20080140451A1 (en) * | 2005-01-10 | 2008-06-12 | Cytori Therapeutics, Inc. | Devices and Methods for Monitoring, Managing, and Servicing Medical Devices |
| US9731045B2 (en) | 2005-04-01 | 2017-08-15 | The Regents Of The University Of Colorado | Shape memory polymer |
| US9271817B2 (en) * | 2005-07-05 | 2016-03-01 | Cook Biotech Incorporated | Tissue augmentation devices and methods |
| US20080248079A1 (en) * | 2005-07-05 | 2008-10-09 | Ryan Dempsey | Tissue augmentation devices and methods |
| US8083726B1 (en) | 2005-09-30 | 2011-12-27 | Advanced Cardiovascular Systems, Inc. | Encapsulating cells and lumen |
| US20080254125A1 (en) * | 2005-10-12 | 2008-10-16 | Thomas Freier | Processing of Chitosan and Chitosan Derivatives |
| WO2007042281A2 (en) | 2005-10-12 | 2007-04-19 | Thomas Freier | Processing of chitosan and chitosan derivatives |
| EP3042930A1 (en) | 2005-10-12 | 2016-07-13 | Thomas Freier | Processing of chitosan and chitosan derivatives |
| US9034379B2 (en) | 2005-10-12 | 2015-05-19 | Thomas Freier | Processing of acylchitosan hydrogels |
| US8414925B2 (en) | 2005-10-12 | 2013-04-09 | Thomas Freier | Processing of acylchitosan hydrogels |
| EP2476726A1 (en) | 2005-10-12 | 2012-07-18 | Thomas Freier | Processing of chitosan and chitosan derivatives |
| US11896505B2 (en) | 2005-10-31 | 2024-02-13 | Scott M. Epstein | Methods for making and using a structural hydrogel polymer device |
| US9180028B2 (en) * | 2005-10-31 | 2015-11-10 | Scott M. Epstein | Structural hydrogel polymer device |
| US10881537B2 (en) | 2005-10-31 | 2021-01-05 | Scott M. Epstein | Structural hydrogel polymer device |
| US20120178874A1 (en) * | 2005-10-31 | 2012-07-12 | Epstein Scott M | Structural hydrogel polymer device |
| US20090248141A1 (en) * | 2006-03-30 | 2009-10-01 | The Regents Of The University Of Colorado | Shape Memory Polymer Medical Devices |
| US20070254041A1 (en) * | 2006-05-01 | 2007-11-01 | Drapeau Susan J | Demineralized bone matrix devices |
| US8039016B2 (en) | 2006-05-01 | 2011-10-18 | Warsaw Orthopedic, Inc. | Malleable implants containing demineralized bone matrix |
| US20100255115A1 (en) * | 2006-05-01 | 2010-10-07 | Warsaw Orthopedic, Inc. | Bone filler material |
| US20100209470A1 (en) * | 2006-05-01 | 2010-08-19 | Warsaw Orthopedic, Inc. An Indiana Corporation | Demineralized bone matrix devices |
| US7838022B2 (en) | 2006-05-01 | 2010-11-23 | Warsaw Orthopedic, Inc | Malleable implants containing demineralized bone matrix |
| US20100209474A1 (en) * | 2006-05-01 | 2010-08-19 | Warsaw Orthopedic, Inc. | Malleable implants containing demineralized bone matrix |
| US9364582B2 (en) | 2006-05-01 | 2016-06-14 | Warsaw Orthopedic, Inc. | Malleable implants containing demineralized bone matrix |
| US8506983B2 (en) | 2006-05-01 | 2013-08-13 | Warsaw Orthopedic, Inc. | Bone filler material |
| US8431147B2 (en) | 2006-05-01 | 2013-04-30 | Warsaw Orthopedic, Inc. | Malleable implants containing demineralized bone matrix |
| US7771741B2 (en) | 2006-05-01 | 2010-08-10 | Warsaw Orthopedic, Inc | Demineralized bone matrix devices |
| US8282953B2 (en) | 2006-05-01 | 2012-10-09 | Warsaw Orthopedic, Inc. | Malleable implants containing demineralized bone matrix |
| US20090304644A1 (en) * | 2006-05-30 | 2009-12-10 | Cytori Therapeutics, Inc. | Systems and methods for manipulation of regenerative cells separated and concentrated from adipose tissue |
| US8597367B2 (en) | 2006-06-16 | 2013-12-03 | Boston Scientific Scimed, Inc. | Partially soluble implantable or insertable medical devices |
| US20080097349A1 (en) * | 2006-06-16 | 2008-04-24 | Boston Scientific Scimed, Inc. | Partially soluble implantable or insertable medical devices |
| US8048168B2 (en) | 2006-06-16 | 2011-11-01 | Boston Scientific Scimed, Inc. | Partially soluble implantable or insertable medical devices |
| US20100015104A1 (en) * | 2006-07-26 | 2010-01-21 | Cytori Therapeutics, Inc | Generation of adipose tissue and adipocytes |
| US20080065192A1 (en) * | 2006-09-13 | 2008-03-13 | Medtronic Vascular, Inc. | Compliance Graded Stent |
| US9585989B2 (en) | 2006-09-19 | 2017-03-07 | Boston Scientific Scimed, Inc. | Ureteral stent having variable hardness |
| US20080071384A1 (en) * | 2006-09-19 | 2008-03-20 | Travis Deal | Ureteral stent having variable hardness |
| US20080077250A1 (en) * | 2006-09-22 | 2008-03-27 | Amos Raymond G | Stent with soluble bladder retention member |
| US20100179666A1 (en) * | 2006-09-22 | 2010-07-15 | Boston Scientific Scimed, Inc. | Stent with soluble bladder retention member |
| WO2008036711A1 (en) * | 2006-09-22 | 2008-03-27 | Boston Scientific Scimed, Inc. | Stent with soluble bladder retention member |
| US8246690B2 (en) | 2006-09-22 | 2012-08-21 | Boston Scientific Scimed, Inc. | Stent with soluble bladder retention member |
| US7713308B2 (en) * | 2006-09-22 | 2010-05-11 | Boston Scientific Scimed, Inc. | Stent with soluble bladder retention member |
| EP2559408A2 (en) | 2006-10-25 | 2013-02-20 | Biosensors International Group, Ltd. | Temporary intraluminal stent and methods of manufacture and use. |
| US20080140106A1 (en) * | 2006-12-12 | 2008-06-12 | Kimberly-Clark Worldwide, Inc. | Enhanced cuff sealing for endotracheal tubes |
| US20080215136A1 (en) * | 2006-12-26 | 2008-09-04 | Gregorich Daniel J | Differential drug release from a medical device |
| US20080287986A1 (en) * | 2007-05-18 | 2008-11-20 | Possis Medical, Inc. | Closure device |
| EP2167144A4 (en) * | 2007-06-13 | 2012-11-21 | Fmc Corp | Biopolymer based implantable degradable devices |
| US20100172953A1 (en) * | 2007-06-13 | 2010-07-08 | Fmc Corporation | Biopolymer Based Implantable Degradable Devices |
| EP2167144A1 (en) * | 2007-06-13 | 2010-03-31 | FMC Corporation | Biopolymer based implantable degradable devices |
| US20100178313A1 (en) * | 2007-06-13 | 2010-07-15 | Fmc Corporation | Implantable Degradable Biopolymer Fiber Devices |
| WO2008157285A1 (en) | 2007-06-13 | 2008-12-24 | Fmc Corporation | Biopolymer based implantable degradable devices |
| US9694110B2 (en) | 2007-12-04 | 2017-07-04 | Boston Scientific Scimed, Inc. | Biodisintegrable medical devices |
| US20090142385A1 (en) * | 2007-12-04 | 2009-06-04 | Warsaw Orthopedic, Inc. | Compositions for treating bone defects |
| US10080819B2 (en) | 2007-12-04 | 2018-09-25 | Warsaw Orthopedic, Inc | Compositions for treating bone defects |
| US20120276221A1 (en) * | 2007-12-04 | 2012-11-01 | Boston Scientific Scimed, Inc. | Biodisintegrable medical devices |
| US10441679B2 (en) | 2007-12-04 | 2019-10-15 | Warsaw Orthopedic, Inc. | Compositions for treating bone defects |
| US9056150B2 (en) | 2007-12-04 | 2015-06-16 | Warsaw Orthopedic, Inc. | Compositions for treating bone defects |
| US20110052503A1 (en) * | 2007-12-21 | 2011-03-03 | Iopharma Technologies Ab | Biodegradable contrast agents |
| US20110008442A1 (en) * | 2008-02-26 | 2011-01-13 | Board Of Regents, The University Of Texas System | Dendritic Macroporous Hydrogels Prepared by Crystal Templating |
| US8728499B2 (en) | 2008-02-26 | 2014-05-20 | Board Of Regents, The University Of Texas System | Dendritic macroporous hydrogels prepared by crystal templating |
| US9896561B2 (en) | 2008-02-26 | 2018-02-20 | Board Of Regents, The University Of Texas System | Dendritic macroporous hydrogels prepared by crystal templating |
| US8668863B2 (en) | 2008-02-26 | 2014-03-11 | Board Of Regents, The University Of Texas System | Dendritic macroporous hydrogels prepared by crystal templating |
| US10982068B2 (en) | 2008-02-26 | 2021-04-20 | Board Of Regents, The University Of Texas System | Dendritic macroporous hydrogels prepared by crystal templating |
| US10442911B2 (en) | 2008-02-26 | 2019-10-15 | Board Of Regents, The University Of Texas System | Dendritic macroporous hydrogels prepared by crystal templating |
| US9320827B2 (en) | 2008-02-26 | 2016-04-26 | Board Of Regents, The University Of Texas System | Dendritic macroporous hydrogels prepared by crystal templating |
| US11760858B2 (en) | 2008-02-26 | 2023-09-19 | Board Of Regents, The University Of Texas System | Dendritic macroporous hydrogels prepared by crystal templating |
| US8840913B2 (en) | 2008-03-27 | 2014-09-23 | Warsaw Orthopedic, Inc. | Malleable multi-component implants and materials therefor |
| US20090246244A1 (en) * | 2008-03-27 | 2009-10-01 | Warsaw Orthopedic, Inc. | Malleable multi-component implants and materials therefor |
| US9730982B2 (en) | 2008-03-27 | 2017-08-15 | Warsaw Orthopedic, Inc. | Malleable multi-component implants and materials therefor |
| WO2009131434A1 (en) * | 2008-04-24 | 2009-10-29 | Universiti Malaya | Extracellular scaffolds comprising blends of polyvinyl alcohol and carboxymethyl chitosan |
| US8574311B2 (en) | 2008-05-07 | 2013-11-05 | Board Of Regents, The University Of Texas System | Versatile biodegradable elastic polymers featured with dual crosslinking mechanism for biomedical applications |
| US20110124765A1 (en) * | 2008-05-07 | 2011-05-26 | Board Of Regents, The University Of Texas System | Versatile Biodegradable Elastic Polymers Featured with Dual Crosslinking Mechanism for Biomedical Applications |
| WO2009137715A3 (en) * | 2008-05-07 | 2010-03-04 | Board Of Regents, The University Of Texas System | Versatile blodegradable elastic polymers featured with dual crosslinking mechanism for biomedical applications |
| WO2009137715A2 (en) * | 2008-05-07 | 2009-11-12 | Board Of Regents, The University Of Texas System | Versatile biodegradable elastic polymers featured with dual crosslinking mechanism for biomedical applications |
| US9486484B2 (en) | 2008-08-19 | 2016-11-08 | Cytori Therapeutics, Inc. | Methods of using adipose tissue-derived cells in the treatment of the lymphatic system and malignant disease |
| US8123739B2 (en) | 2008-08-19 | 2012-02-28 | Cook Medical Technologies Llc | Drainage catheter and method for catheterizing a patient |
| US8784801B2 (en) | 2008-08-19 | 2014-07-22 | Cytori Therapeutics, Inc. | Methods of using adipose tissue-derived cells in the treatment of the lymphatic system and malignant disease |
| US20110206646A1 (en) * | 2008-08-19 | 2011-08-25 | Zeni Alfonso | Methods of using adipose tissue-derived cells in the treatment of the lymphatic system and malignant disease |
| US8591672B2 (en) | 2009-01-08 | 2013-11-26 | Bio Dg, Inc. | Implantable medical devices comprising bio-degradable alloys |
| WO2010080932A2 (en) | 2009-01-08 | 2010-07-15 | Bio Dg, Inc. | Implantable medical devices comprising bio-degradable alloys |
| US8246762B2 (en) | 2009-01-08 | 2012-08-21 | Bio Dg, Inc. | Implantable medical devices comprising bio-degradable alloys |
| US20100174367A1 (en) * | 2009-01-08 | 2010-07-08 | Bio Dg, Inc | Implantable medical devices comprising bio-degradable alloys |
| EP3505139A1 (en) | 2009-01-08 | 2019-07-03 | Bio DG, Inc. | Implantable medical devices comprising bio-degradable alloys |
| US9808252B2 (en) | 2009-04-02 | 2017-11-07 | Endoshape, Inc. | Vascular occlusion devices |
| US9133431B2 (en) | 2009-05-01 | 2015-09-15 | Bimini Technologies Llc | Systems, methods and compositions for optimizing tissue and cell enriched grafts |
| US20100279405A1 (en) * | 2009-05-01 | 2010-11-04 | Alvin Peterson | Systems, methods and compositions for optimizing tissue and cell enriched grafts |
| US10046085B2 (en) | 2009-05-29 | 2018-08-14 | Incube Labs, Llc | Biodegradable medical implants, polymer compositions and methods of use |
| US11642438B2 (en) | 2009-05-29 | 2023-05-09 | Incube Labs, Llc | Biodegradable medical implants, polymer compositions and methods of use |
| WO2010138212A3 (en) * | 2009-05-29 | 2011-04-21 | Incube Labs, Llc | Biodegradable medical implants, polymer compositions and methods of use |
| WO2010138212A2 (en) * | 2009-05-29 | 2010-12-02 | Incube Labs, Llc | Biodegradable medical implants, polymer compositions and methods of use |
| US9510931B2 (en) | 2009-11-24 | 2016-12-06 | Covidien Lp | Reinforced tissue patch |
| US8690960B2 (en) | 2009-11-24 | 2014-04-08 | Covidien Lp | Reinforced tissue patch |
| US20110125287A1 (en) * | 2009-11-24 | 2011-05-26 | Tyco Healthcare Group LP, New Haven CT and Confluent Surgical, Inc. | Reinforced Tissue Patch |
| US20110130774A1 (en) * | 2009-11-30 | 2011-06-02 | Tyco Healthcare Group Lp | Ventral Hernia Repair With Barbed Suture |
| US9398943B2 (en) | 2009-11-30 | 2016-07-26 | Covidien Lp | Ventral hernia repair with barbed suture |
| EP2600800A4 (en) * | 2010-08-05 | 2014-01-22 | Taris Biomedical Inc | Ureteral stent drug delivery device, kit, and method |
| EP2600800A1 (en) * | 2010-08-05 | 2013-06-12 | Taris Biomedical, Inc. | Ureteral stent drug delivery device, kit, and method |
| US9492266B2 (en) | 2010-08-05 | 2016-11-15 | Taris Biomedical Llc | Ureteral stent drug delivery device, kit, and method |
| US9656001B2 (en) | 2010-10-08 | 2017-05-23 | Board Of Regents, The University Of Texas System | Anti-adhesive barrier membrane using alginate and hyaluronic acid for biomedical applications |
| US11890344B2 (en) | 2010-10-08 | 2024-02-06 | Board Of Regents, The University Of Texas System | One-step processing of hydrogels for mechanically robust and chemically desired features |
| US10279042B2 (en) | 2010-10-08 | 2019-05-07 | Board Of Regents, The University Of Texas System | One-step processing of hydrogels for mechanically robust and chemically desired features |
| US9095558B2 (en) | 2010-10-08 | 2015-08-04 | Board Of Regents, The University Of Texas System | Anti-adhesive barrier membrane using alginate and hyaluronic acid for biomedical applications |
| US8946194B2 (en) | 2010-10-08 | 2015-02-03 | Board Of Regents, University Of Texas System | One-step processing of hydrogels for mechanically robust and chemically desired features |
| US11058802B2 (en) | 2010-10-08 | 2021-07-13 | Board Of Regents, The University Of Texas System | Anti-adhesive barrier membrane using alginate and hyaluronic acid for biomedical applications |
| US11246937B2 (en) | 2010-10-08 | 2022-02-15 | Board Of Regents, The University Of Texas System | One-step processing of hydrogels for mechanically robust and chemically desired features |
| US10314950B2 (en) | 2010-10-08 | 2019-06-11 | Board Of Regents, The University Of Texas System | Anti-adhesive barrier membrane using alginate and hyaluronic acid for biomedical applications |
| US11857701B2 (en) | 2010-10-08 | 2024-01-02 | Board Of Regents, The University Of Texas System | Anti-adhesive barrier membrane using alginate and hyaluronic acid for biomedical applications |
| US11744926B2 (en) | 2010-10-08 | 2023-09-05 | Board Of Regents, The University Of Texas System | Anti-adhesive barrier membrane using alginate and hyaluronic acid for biomedical applications |
| US10272176B2 (en) | 2011-03-07 | 2019-04-30 | The Regents Of The University Of Colorado | Shape memory polymer intraocular lenses |
| US10286106B2 (en) | 2011-03-07 | 2019-05-14 | The Regents Of The University Of Colorado | Intraocular lenses |
| US10286107B2 (en) | 2011-03-07 | 2019-05-14 | The Regents Of The University Of Colorado, A Body Corporate | Shape memory polymer intraocular lenses |
| US10286105B2 (en) | 2011-03-07 | 2019-05-14 | The Regents Of The University Of Colorado, A Body Corporate | Shape memory polymer intraocular lenses |
| US9427493B2 (en) | 2011-03-07 | 2016-08-30 | The Regents Of The University Of Colorado | Shape memory polymer intraocular lenses |
| US8968927B2 (en) | 2011-07-15 | 2015-03-03 | Covidien Lp | Degradable implantable battery |
| US9362571B2 (en) | 2011-07-15 | 2016-06-07 | Covidien Lp | Degradable implantable battery |
| US8968926B2 (en) | 2011-07-15 | 2015-03-03 | Covidien Lp | Degradable implantable galvanic power source |
| US10201351B2 (en) | 2011-09-30 | 2019-02-12 | Endoshape, Inc. | Continuous embolic coil and methods and devices for delivery of the same |
| US10603043B2 (en) | 2012-01-17 | 2020-03-31 | Endoshape, Inc. | Occlusion device for a vascular or biological lumen |
| US9675789B2 (en) * | 2012-06-29 | 2017-06-13 | National Cheng Kung University | Embeddable micro-needle patch for transdermal drug delivery and method of manufacturing the same |
| US20140005606A1 (en) * | 2012-06-29 | 2014-01-02 | Mei-Chin Chen | Embeddable micro-needle patch for transdermal drug delivery and method of manufacturing the same |
| US11565027B2 (en) | 2012-12-11 | 2023-01-31 | Board Of Regents, The University Of Texas System | Hydrogel membrane for adhesion prevention |
| US9662424B2 (en) | 2012-12-11 | 2017-05-30 | Board Of Regents, The University Of Texas System | Hydrogel membrane for adhesion prevention |
| US10272184B2 (en) | 2012-12-11 | 2019-04-30 | Board Of Regents, The University Of Texas System | Hydrogel membrane for adhesion prevention |
| US10850011B2 (en) | 2012-12-11 | 2020-12-01 | Board Of Regents, The University Of Texas | Hydrogel membrane for adhesion prevention |
| US9445884B2 (en) | 2013-01-30 | 2016-09-20 | Boston Scientific Scimed, Inc. | Ureteral stent with drug-releasing structure |
| WO2014120587A1 (en) * | 2013-01-30 | 2014-08-07 | Boston Scientific Scimed, Inc. | Ureteral stent with drug-releasing structure |
| US11478570B2 (en) | 2013-03-14 | 2022-10-25 | Bio Dg, Inc. | Implantable medical devices comprising bio-degradable alloys with enhanced degradation rates |
| EP3610894A1 (en) | 2013-03-14 | 2020-02-19 | Bio DG, Inc. | Implantable medical devices comprising bio-degradable alloys with enhanced degradation rates |
| WO2014153144A1 (en) | 2013-03-14 | 2014-09-25 | Radisch Herbert R | Implantable medical devices comprising bio-degradable alloys with enhanced degradation rates |
| US10765775B2 (en) | 2013-03-14 | 2020-09-08 | Bio Dg, Inc. | Implantable medical devices comprising bio-degradable alloys with enhanced degradation rates |
| US11285304B2 (en) | 2013-03-15 | 2022-03-29 | Taris Biomedical Llc | Drug delivery devices with drug-permeable component and methods |
| US10286199B2 (en) | 2013-03-15 | 2019-05-14 | Taris Biomedical Llc | Drug delivery devices with drug-permeable component and methods |
| US10315019B2 (en) | 2013-03-15 | 2019-06-11 | Taris Biomedical Llc | Drug delivery devices with drug-permeable component and methods |
| EP2870981A1 (en) | 2013-11-12 | 2015-05-13 | Covidien LP | Degradable implantable battery |
| WO2015191853A1 (en) * | 2014-06-13 | 2015-12-17 | Siemens Healthcare Diagnostics Inc. | Sample delivery system |
| US10391484B2 (en) | 2014-06-13 | 2019-08-27 | Siemens Healthcare Diagnostics Inc. | Sample delivery system |
| WO2016033424A1 (en) | 2014-08-29 | 2016-03-03 | Genzyme Corporation | Methods for the prevention and treatment of major adverse cardiovascular events using compounds that modulate apolipoprotein b |
| US10010400B2 (en) | 2015-03-30 | 2018-07-03 | Taris Biomedical Llc | Devices and methods for local delivery of drug to upper urinary tract |
| US11744998B2 (en) | 2015-04-23 | 2023-09-05 | Taris Biomedical Llc | Drug delivery devices with drug-permeable component and methods |
| US10894150B2 (en) | 2015-04-23 | 2021-01-19 | Taris Biomedical Llc | Drug delivery devices with drug-permeable component and methods |
| WO2016181371A1 (en) * | 2015-05-14 | 2016-11-17 | Association For The Advancement Of Tissue Engineering And Cell Based Technologies And Therapies - A4Tec | An ureteral stent, methods and uses thereof |
| JP2018520818A (en) * | 2015-05-14 | 2018-08-02 | アソシエーション フォー ジ アドバンスメント オブ ティシュー エンジニアリング アンド セル ベイスト テクノロジーズ アンド セラピーズ(エイ4テック)− アソシアソンAssociation For The Advancement Of Tissue Engineering And Cell Based Technologies & Therapies (A4Tec) − Associacao | Ureteral stent, method and use thereof |
| US11000633B2 (en) | 2015-05-14 | 2021-05-11 | Association For The Advancement Of Tissue Engineer | Ureteral stent, methods and uses thereof |
| CN109195642B (en) * | 2016-06-07 | 2021-12-10 | 医药研究有限公司 | Filling composition for tissue enhancement comprising nucleic acid, chitosan and hyaluronic acid and preparation method thereof |
| CN109195642A (en) * | 2016-06-07 | 2019-01-11 | 医药研究产品有限公司 | Tissue enhancing filled compositions comprising nucleic acid, chitosan and hyaluronic acid and preparation method thereof |
| CN111511310A (en) * | 2017-12-22 | 2020-08-07 | 聚合-医药有限公司 | Tubular implant with controlled biodegradation |
| JP2021509829A (en) * | 2017-12-22 | 2021-04-08 | ポリ−メド インコーポレイテッド | Tubular implant with controlled biodegradation |
| CN111511310B (en) * | 2017-12-22 | 2023-11-28 | 聚合-医药有限公司 | Tubular implant with controlled biodegradation |
| CN110721391A (en) * | 2019-09-30 | 2020-01-24 | 深圳泰睿仕医疗科技有限公司 | Disposable ureter catheterization support tube |
| WO2021148564A1 (en) * | 2020-01-22 | 2021-07-29 | Dsm Ip Assets B.V. | Method of cross-linking biomaterial with a polyfunctional aziridine compound and products obtained therewith |
| CN115141447B (en) * | 2021-03-30 | 2023-09-15 | 合肥杰事杰新材料股份有限公司 | Antibacterial polyhydroxyethyl methacrylate material and preparation method and application thereof |
| CN115141447A (en) * | 2021-03-30 | 2022-10-04 | 合肥杰事杰新材料股份有限公司 | Antibacterial poly (hydroxyethyl methacrylate) material and preparation method and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6368356B1 (en) | Medical devices comprising hydrogel polymers having improved mechanical properties | |
| US6387978B2 (en) | Medical devices comprising ionically and non-ionically crosslinked polymer hydrogels having improved mechanical properties | |
| EP2363104B1 (en) | Hydrogels that undergo volumetric expansion in response to changes in their environment and their methods of manufacture and use | |
| EP1496814B1 (en) | Resorption-controllable medical implants | |
| US6096018A (en) | Medical treatment utilizing ionically crosslinked medical devices | |
| US8318195B2 (en) | Controlling resorption of bioresorbable medical implant material by application of microwave, ultrasound or radiofrequencies | |
| JP4331795B2 (en) | Medical device with improved elastic response | |
| WO2000050103A1 (en) | Medical devices comprising hydrogel polymers having improved mechanical properties | |
| AU2002306707A1 (en) | Controlled resorption of medical implants | |
| AU2007234593B2 (en) | Controlled resorption of medical implants |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: SCIMED LIFE SYSTEMS, INC., MINNESOTA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:ZHONG, SHENG PING;MADENJIAN, ARTHUR R.;GODSHALL, DOUGLAS E.;REEL/FRAME:012636/0837;SIGNING DATES FROM 20020102 TO 20020117 Owner name: SCIMED LIFE SYSTEMS, INC., MINNESOTA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BOSTON SCIENTIFIC SCIMED INC.;REEL/FRAME:012638/0115 Effective date: 19990727 |
|
| AS | Assignment |
Owner name: BOSTON SCIENTIFIC SCIMED, INC., MINNESOTA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:HERCULES INCORPORATED;REEL/FRAME:012647/0674 Effective date: 19990511 Owner name: HERCULES INCORPORATED, DELAWARE Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:RONAN, JOHN M.;THOMPSON, SAMUEL A.;REEL/FRAME:012647/0680 Effective date: 19960821 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| AS | Assignment |
Owner name: BOSTON SCIENTIFIC SCIMED, INC., MINNESOTA Free format text: CHANGE OF NAME;ASSIGNOR:SCIMED LIFE SYSTEMS, INC.;REEL/FRAME:018505/0868 Effective date: 20050101 Owner name: BOSTON SCIENTIFIC SCIMED, INC.,MINNESOTA Free format text: CHANGE OF NAME;ASSIGNOR:SCIMED LIFE SYSTEMS, INC.;REEL/FRAME:018505/0868 Effective date: 20050101 |
|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| FPAY | Fee payment |
Year of fee payment: 12 |